High-temperature-resistant glucose isomerase mutant and application thereof
A technology of glucose isomerase and mutant is applied in the application field of isomerizing glucose to produce ultra-high D-fructose concentration and high fructose syrup, and achieves the effect of significant technical progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
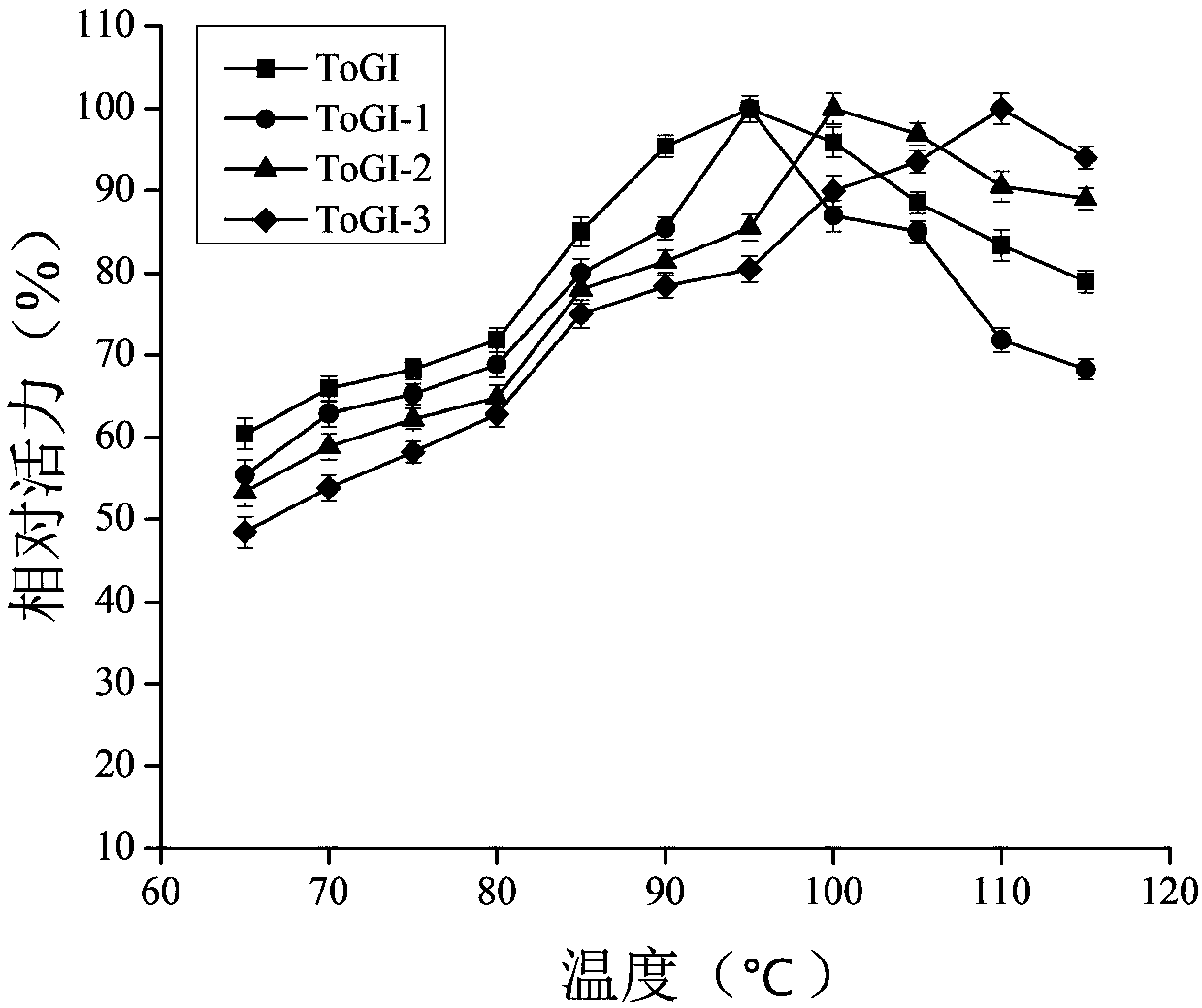
[0020] Example 1: Construction and screening of ToGI single point mutants
[0021] 1. Mutant construction
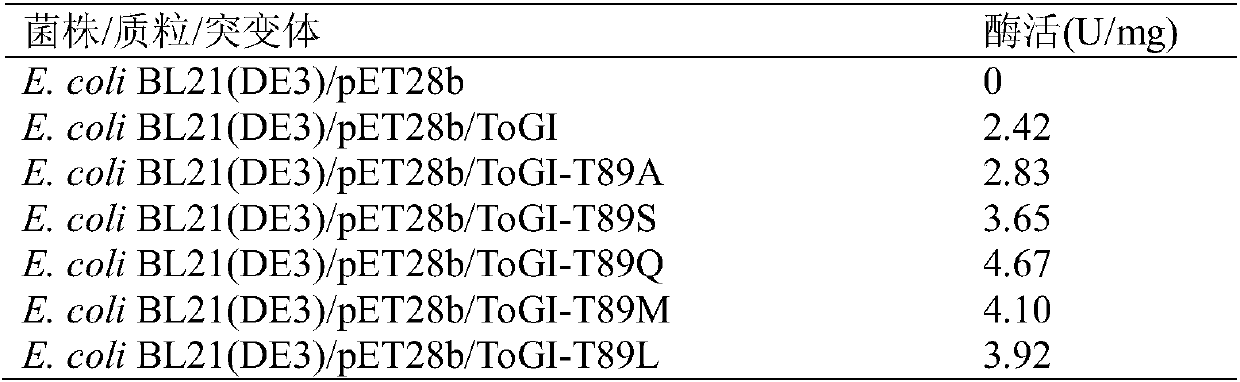
[0022] According to the parental sequence of ToGI (the amino acid sequence is shown in SEQ ID NO.1, and the nucleotide sequence is shown in SEQ ID NO.2), the mutation primers for site-directed mutation were designed, and the recombinant vector pET28b / ToGI was used as a template by using rapid PCR technology. To introduce a single mutation at position 89, the primers are:
[0023] Forward primer CCGATGGTT NNN GCTAACCTGTTC (the underline is the mutated base)
[0024] reverse primer CAGGTTAGC NNN AACCATCGGAACTTTC (the underline is the mutated base)
[0025] PCR reaction system: 2×Phanta Max Buffer (containing Mg 2+ ) 25μL, dNTPs 10mM, forward primer 2μL, reverse primer 2μL, template DNA 1μL, Phanta Max Super-Fidelity DNA Polymerase 50U, add ddH 2 0 to 50 μL.
[0026] The PCR amplification conditions were 95°C for 3min; (95°C for 15s, 50°C for 15s, 61°C for 6.5min) ...
Embodiment 2
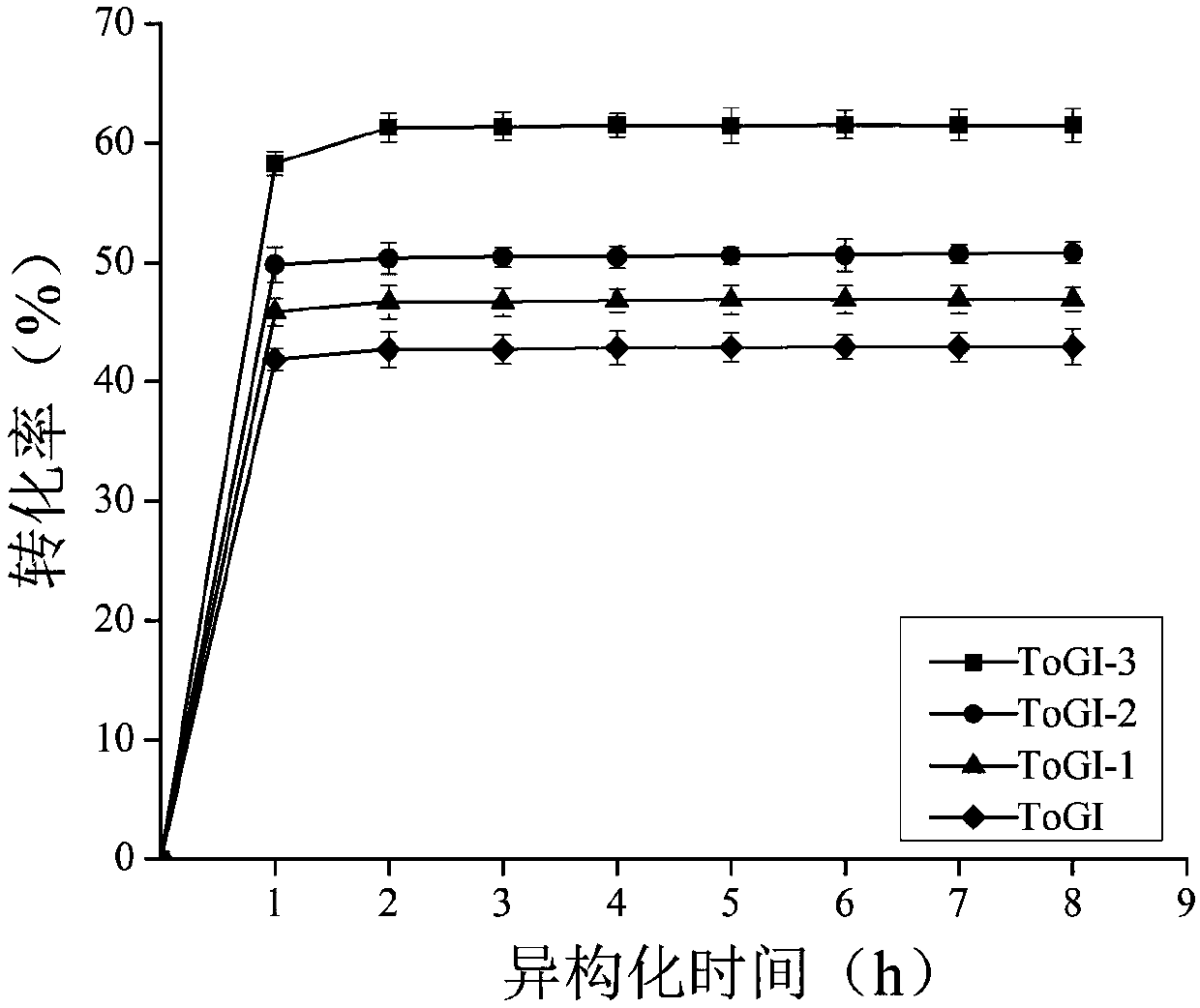
[0037] Example 2: Construction and screening of two-site mutants of glucose isomerase
[0038] According to the single mutant ToGI-1 sequence constructed in Example 1, the mutation primers for site-directed mutation were designed, and the rapid PCR technology was used to use the recombinant vector pET28b / ToGI-1 as a template to introduce a single mutation at position 136. The primers were:
[0039] Forward primer CGTTGTT NNN CCGGGTCGTG (the underline is the mutated base)
[0040] reverse primer GACCCGG NNN AACAACGTAGATTTC (the underline is the mutated base)
[0041] PCR reaction system: 2×Phanta Max Buffer (containing Mg 2+ ) 25μL, dNTPs 10mM, forward primer 2μL, reverse primer 2μL, template DNA 1μL, Phanta Max Super-Fidelity DNA Polymerase 50U, add ddH 2 0 to 50 μL.
[0042] The PCR amplification conditions were 95°C for 3min; (95°C for 15s, 50°C for 15s, 62°C for 6.5min) for 30 cycles; 72°C for 5min.
[0043] The PCR product was transformed into E.coliBL21 (DE3) comp...
Embodiment 3
[0049] Example 3: Construction and screening of three-site mutants of glucose isomerase
[0050] According to the mutant ToGI-2 sequence constructed in Example 2, the mutation primers for site-directed mutagenesis were designed, using the rapid PCR technology, using the recombinant vector pET28b / ToGI-2 as a template, and introducing a single mutation at position 352, the primers were:
[0051] Forward primer CGTGCT NNN GCTCTGAAAG (the underline is the mutated base)
[0052] reverse primer CAGAGC NNN AGCACGTTCAC (the underline is the mutated base)
[0053] PCR reaction system: 2×Phanta Max Buffer (containing Mg 2+ ) 25μL, dNTPs 10mM, forward primer 2μL, reverse primer 2μL, template DNA 1μL, Phanta Max Super-Fidelity DNA Polymerase 50U, add ddH 2 0 to 50 μL.
[0054] The PCR amplification conditions were 95°C for 3min; (95°C for 15s, 50°C for 15s, 60°C for 6.5min) 30 cycles; 72°C for 5min.
[0055] The PCR product was transformed into E.coliBL21 (DE3) competent cells, an...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap