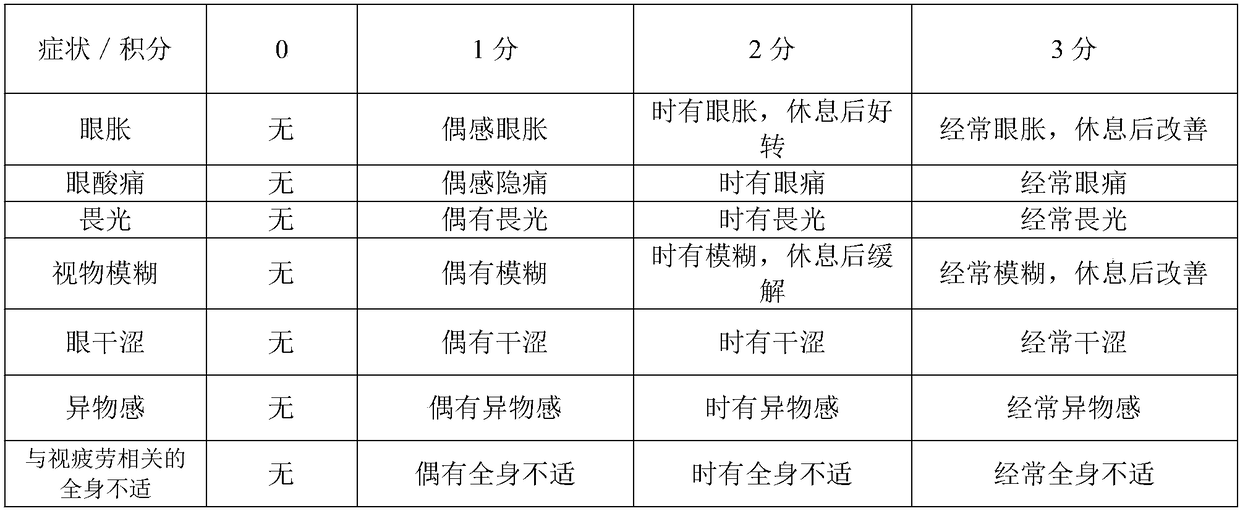
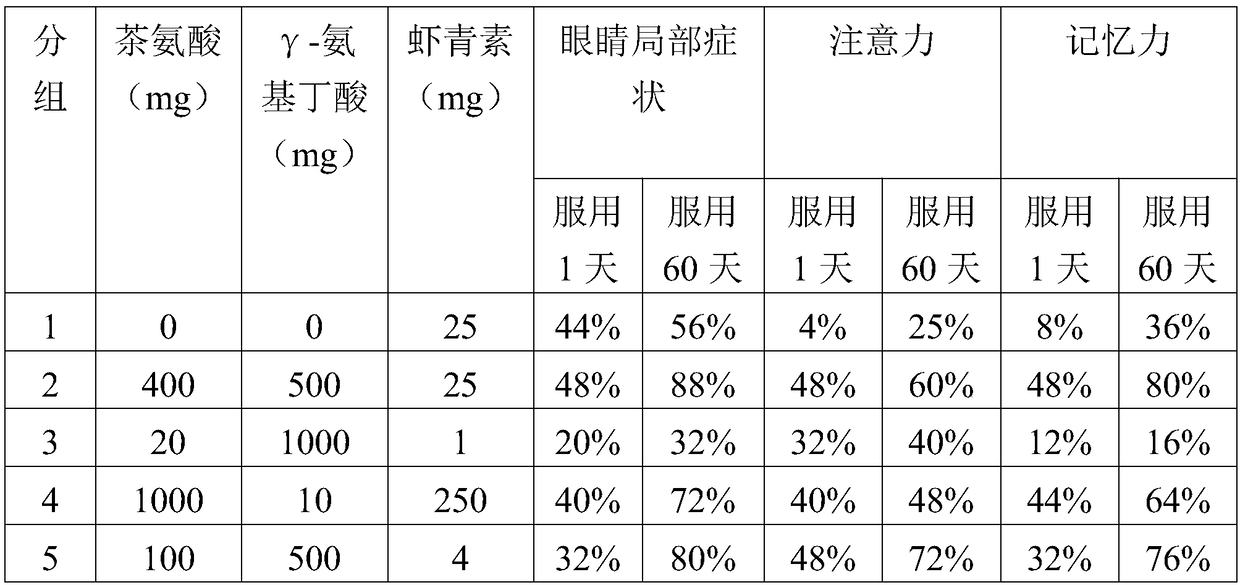
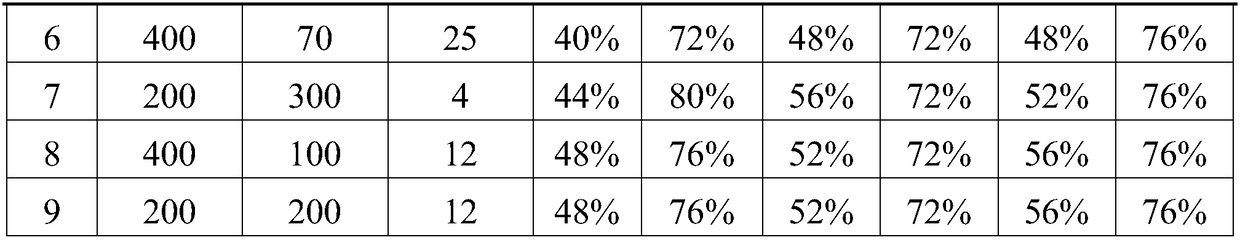
Composition containing theanine, gamma-aminobutyric acid and astaxanthin for relieving asthenopia
A technology for relieving visual fatigue and GABA, applied in the field of medicine and health, can solve problems such as memory loss, inattention, and ineffective improvement of local eye symptoms, and achieve the effect of improving systemic symptoms and broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 composition of the present invention
[0032] Raw material weight ratio: 200-400 parts of theanine, 100-300 parts of γ-aminobutyric acid, 4-12 parts of astaxanthin, 100-400 parts of DHA, 5-15 parts of lutein ester, (3R, 3' 1-5 parts of R)-dihydroxy-β-carotene.
[0033] Preparation:
[0034] 1. Mix theanine, γ-aminobutyric acid, astaxanthin, DHA, (3R,3'R)-dihydroxy-β-carotene and lutein ester in a weight ratio, then add syrup and stir evenly.
[0035] 2. After the injection mold is cooled and formed, it can be made into hard, toffee, sandwich, crispy, burnt (toffee), inflated, gel, gum base, compressed, liquid, diaphragm, fancy and other candies as required types, as well as chocolate and chocolate products, cocoa butter substitute chocolate and cocoa butter substitute chocolate products, jelly (juice type, pulp type, fruity type, milk type, etc.) and other confectionary products.
Embodiment 2
[0036] The preparation of embodiment 2 compositions of the present invention
[0037] Raw material weight ratio: 200-400 parts of theanine, 100-300 parts of γ-aminobutyric acid, 4-25 parts of astaxanthin, 100-400 parts of DHA, (3R,3'R)-dihydroxy-β-carrot 1-10 parts of lutein, 1-25 parts of lutein ester.
[0038] Preparation method: select the raw materials of theanine, γ-aminobutyric acid, astaxanthin, (3R,3'R)-dihydroxy-β-carotene, and lutein ester to mix, add the required treated water and 30- 45 The liquid milk is fully stirred, dissolved, and homogenized, and the homogenization pressure is 25Mpa, and then subjected to ultra-high temperature sterilization, aseptic packaging, and put into storage after passing the inspection.
Embodiment 3
[0039] The preparation of embodiment 3 compositions of the present invention
[0040] Raw material weight ratio: 200-600 parts of theanine, 100-400 parts of γ-aminobutyric acid, 1-25 parts of astaxanthin, 100-600 parts of DHA, (3R,3'R)-dihydroxy-β-carrot 0.2-10 parts of lutein, 0.6-25 parts of lutein ester.
[0041] Preparation method: select theanine, γ-aminobutyric acid, astaxanthin, DHA, (3R,3'R)-dihydroxy-β-carotene, lutein ester, stir evenly, sterilize with ozone, fill, It can be taken directly or added to other foods as a functional auxiliary material.
[0042] The beneficial effects of the present invention are demonstrated by test examples below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com