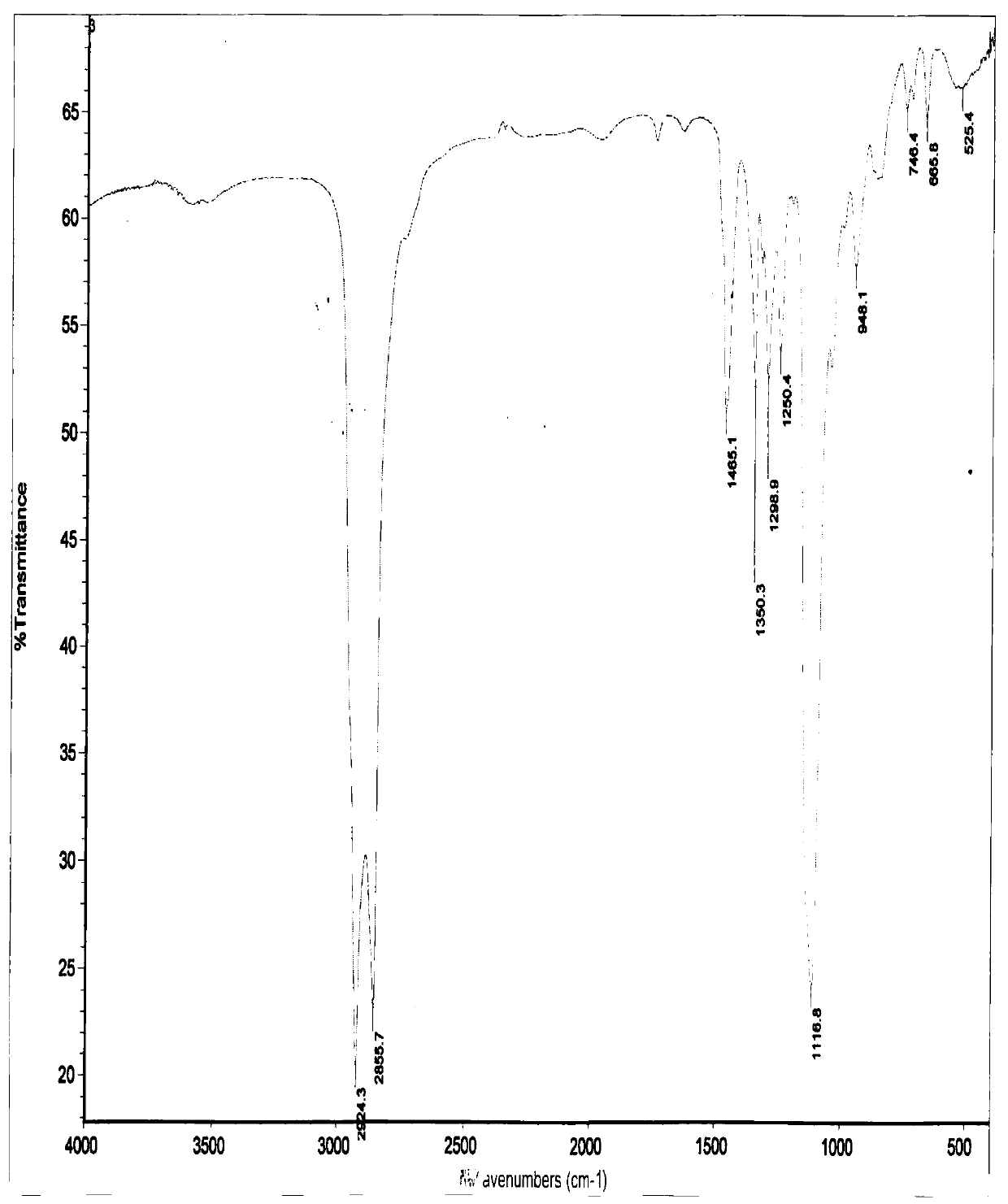
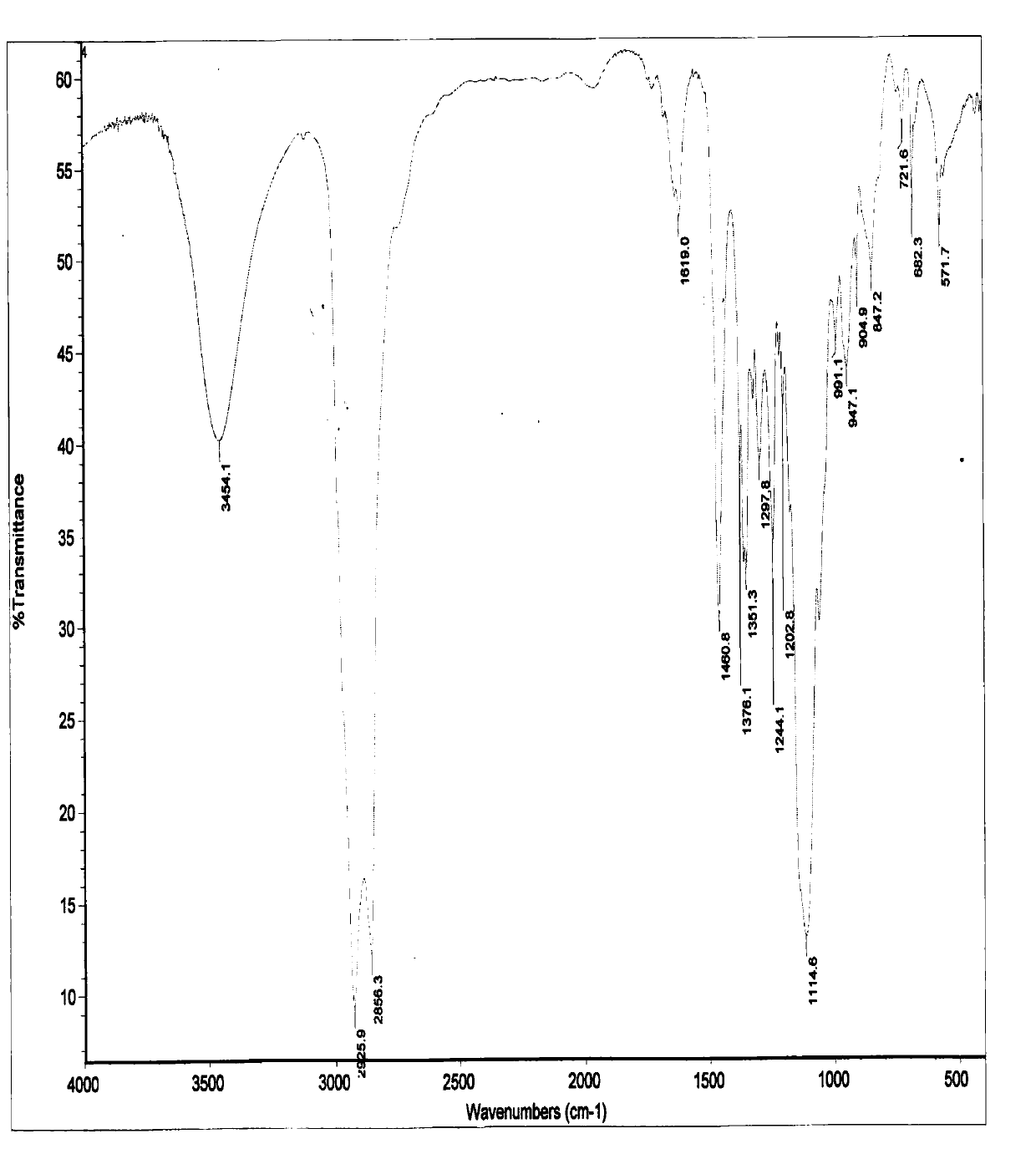
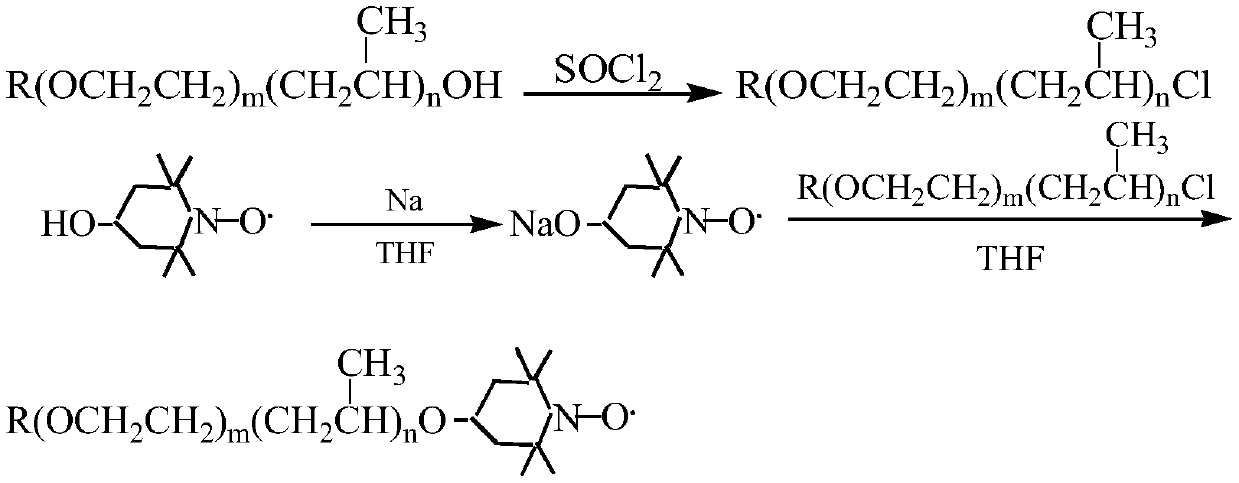
A kind of preparation method of nitroxide free radical spin probe with surface activity
A technology of nitroxide free radicals and surface activity, which is applied in the direction of organic chemistry, can solve the problems of inconvenience, increased use cost, nitroxide free radical spin probes without surface activity, etc., and achieve the effect of separation and purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This embodiment is realized through the following steps:
[0028] (1), preparation of intermediate-dodecyl alcohol polyoxyethylene ether (m=9) chloride
[0029] Add 0.0205mol dodecyl alcohol polyoxyethylene ether (m=9) and 0.0369mol pyridine into a 100mL three-necked flask equipped with a stirrer, reflux condenser, and thermometer, stir evenly and slowly add 0.0369mol thionyl chloride dropwise, Stir the reaction at 60-70°C for 10 hours, stop the reaction, cool, and let it stand, the organic matter in the upper layer is neutralized with 15% sodium hydroxide solution to pH ≥ 7, and then transferred to a separatory funnel to separate the water layer, and the organic layer is washed with water for 5 -6 times, until there is no irritating smell; then pour it into a single-necked round bottom flask, add 50ml of petroleum ether (60-90°C), reflux with a water separator to remove water, and then distill the petroleum ether to obtain a brown transparent liquid .
[0030] Depend...
Embodiment 2
[0036] This embodiment is realized through the following steps:
[0037] (1), preparation of intermediate-octylphenol polyoxyethylene ether (m=10) chloride
[0038] Add 0.0205mol octylphenol polyoxyethylene ether (m=10) and 0.0369mol pyridine into a 100mL three-necked flask equipped with a stirrer, reflux condenser, and thermometer, stir evenly, and slowly add 0.0369mol thionyl chloride dropwise, Stir the reaction at 60-70°C for 10 hours, stop the reaction, cool, and let it stand, the organic matter in the upper layer is neutralized with 15% sodium hydroxide solution to pH ≥ 7, and then transferred to a separatory funnel to separate the water layer, and the organic layer is washed with water for 5 -6 times, until there is no irritating smell; then pour it into a single-necked round bottom flask, add 50ml of petroleum ether (60-90°C), reflux with a water separator to remove water, and then distill the petroleum ether to obtain a brown transparent liquid .
[0039] (2), prepar...
Embodiment 3
[0043] This embodiment is realized through the following steps:
[0044] (1), preparation of intermediate-fatty alcohol polyoxypropylene ether (n=15) chloride
[0045] Add 0.0205mol fatty alcohol polyoxypropylene ether (n=15), 0.0369mol pyridine in a 100mL three-necked flask equipped with a stirrer, reflux condenser, and thermometer, and slowly add 0.0369mol thionyl chloride dropwise after stirring evenly. Stir and react at 60-70°C for 3.5 hours, stop the reaction, cool, and let stand, and neutralize the organic matter in the upper layer with 15% sodium hydroxide solution to pH ≥ 7, transfer it into a separatory funnel to separate the water layer, and wash the organic layer with water for 5- 6 times until there is no irritating smell; then pour it into a single-necked round bottom flask, add 50ml of petroleum ether (60-90°C), reflux with a water separator to remove water, and then distill the petroleum ether to obtain a brown transparent liquid.
[0046](2), preparation of su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com