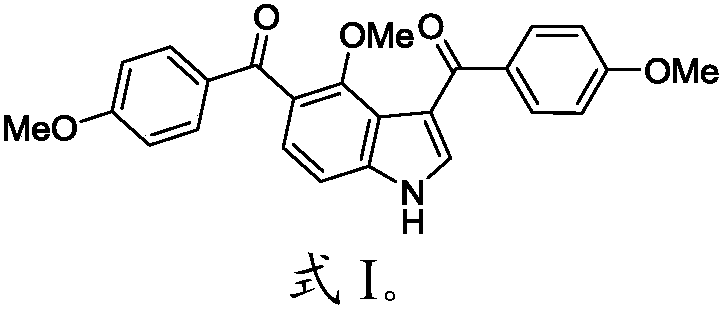
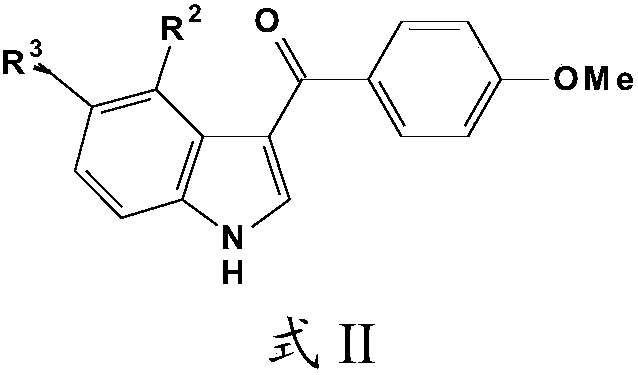
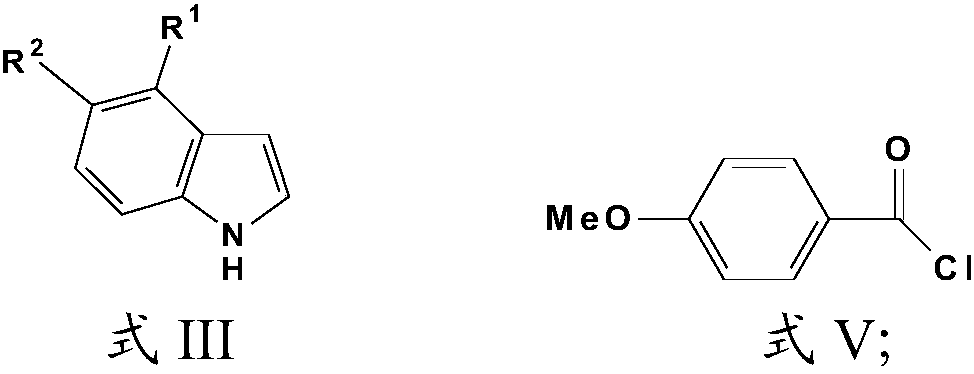Compound and preparation method thereof as well as application in preparation of tumor drug resistance reversal agent
A drug resistance reversal and compound technology, which is applied in the field of medicine, can solve the problems of low yield, difficult to meet clinical application, high production cost, etc., and achieve the effect of simple reaction, drug resistance reversal and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 compound (5-methoxyl-1H-indol)-3-(4-methoxyphenyl) ketone (5-Methoxy-1H-indol-3-yl) (4-methoxyphenyl)methanone ( 1c) and its synthesis
[0043]
[0044] 588mg compound of formula III, 1360mg compound of formula V, POCl of 5ml 3 ; 2 g of AlCl 3 , added in 10mL 1,2-dichloroethane, reacted at 60°C for 4h, cooled down to room temperature after the reaction, dropped into ice water, distilled with CH 2 Cl 2 The product was extracted and separated by column chromatography with a total yield of 40% and a purity of 99.5%.
[0045]IR spectrum (KBr coating cm -1 ):3151,2956,1602,1427,1026cm -1 ;
[0046] 1 H NMR spectrum (ppm): δ (ppm) 11.90 (br, 1H), 7.89 (d, J = 3.2Hz, 1H), 7.80 (d, J = 8.8Hz2H), 7.76 (d, J = 2.4Hz, 1H ),7.40(d,J=8.8Hz,1H),7.07(d,J=8.8Hz 2H),6.88(dd,J=8.8,2.4Hz,1H),3.85(s,3H),3.80(s, 3H).(solvent DMSO-d 6 , TMS internal standard)
Embodiment 2
[0047] Example 2 (4-methoxy-1H-indole)-3,5-bis(4-methoxyphenyl)methanone (4-methoxy-1H-indole-3,5-diyl)bis(( 4-methoxyphenyl)methanone)(2f) and its synthesis
[0048]
[0049] 588mg compound of formula III, 1360mg compound of formula V, POCl of 5mL 3 ; 2 g of AlCl 3 , added in 10mL 1,2-dichloroethane, reacted at 60°C for 4h, cooled down to room temperature after the reaction, dropped into ice water, distilled with CH 2 Cl 2 The product was extracted and separated by column chromatography with a total yield of 19% and a purity of 96.7%.
[0050] IR spectrum (KBr coating cm -1 ):3380,2935,1600,1361,1075cm -1 ;
[0051] 1 H NMR spectrum (ppm) 12.00 (br, 1H), 7.77 (dd, J = 8.8, 2.4Hz, 4H), 7.60-7.56 (m, 2H), 7.13 (dd, J = 8.8, 2.0Hz, 2H), 7.05(dd, J=8.8,2.0Hz,2H),6.78(d,J=8.8Hz,1H),3.89(s,3H),3.86(s,3H).(solvent DMSO-d 6 , TMS internal standard)
[0052] Drug efficacy verification
[0053] 1. Cell culture method
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com