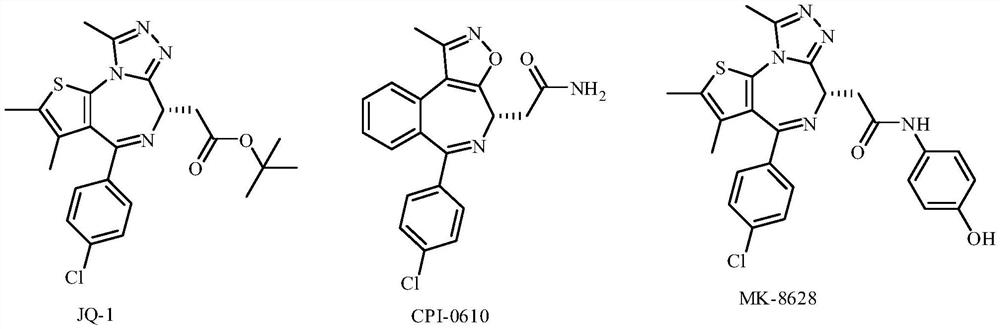
Heteroaromatic ring derivatives
A drug and compound technology, applied in the field of medicine, can solve problems such as short patent protection period and poor drug metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
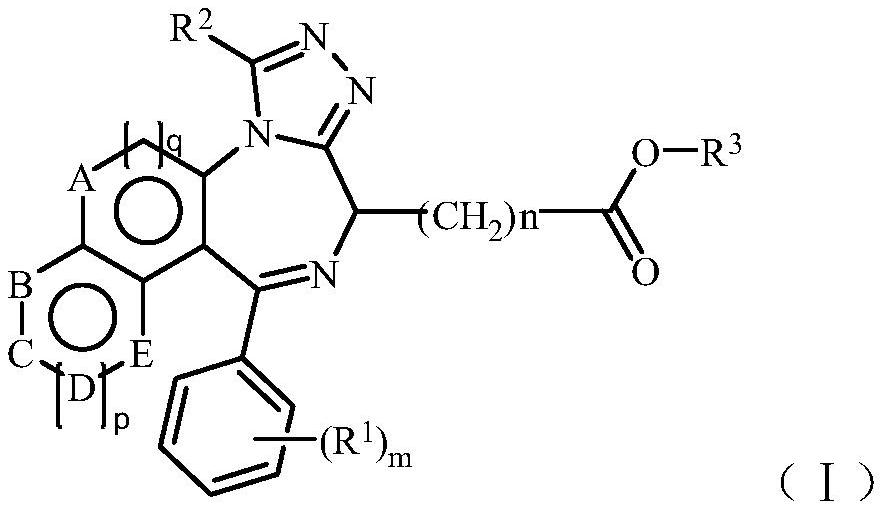
[0069] The preparation method of the compound of the general formula (I) of the present invention comprises making the compound shown in the general formula (IV), its pharmaceutically acceptable salt or its easily hydrolyzed ester, and the compound shown in the general formula (V), its pharmaceutically acceptable Accepted salts, their readily hydrolyzable esters, or their isomers react,
[0070]
[0071] Among them, R 1 , R 2 , R 3 , A, B, C, D, E, m, n, p and q are as defined above.
[0072] The above-mentioned compounds of the present invention can be synthesized by the methods described in the following schemes and / or other techniques known to those of ordinary skill in the art, but are not limited to the following methods.
[0073] When n=1, the reaction scheme is:
[0074]
[0075] Reaction steps:
[0076] Step 1: Preparation of compound b
[0077] The raw material a was added into aqueous hydrobromic acid solution, refluxed overnight, and the reaction was alm...
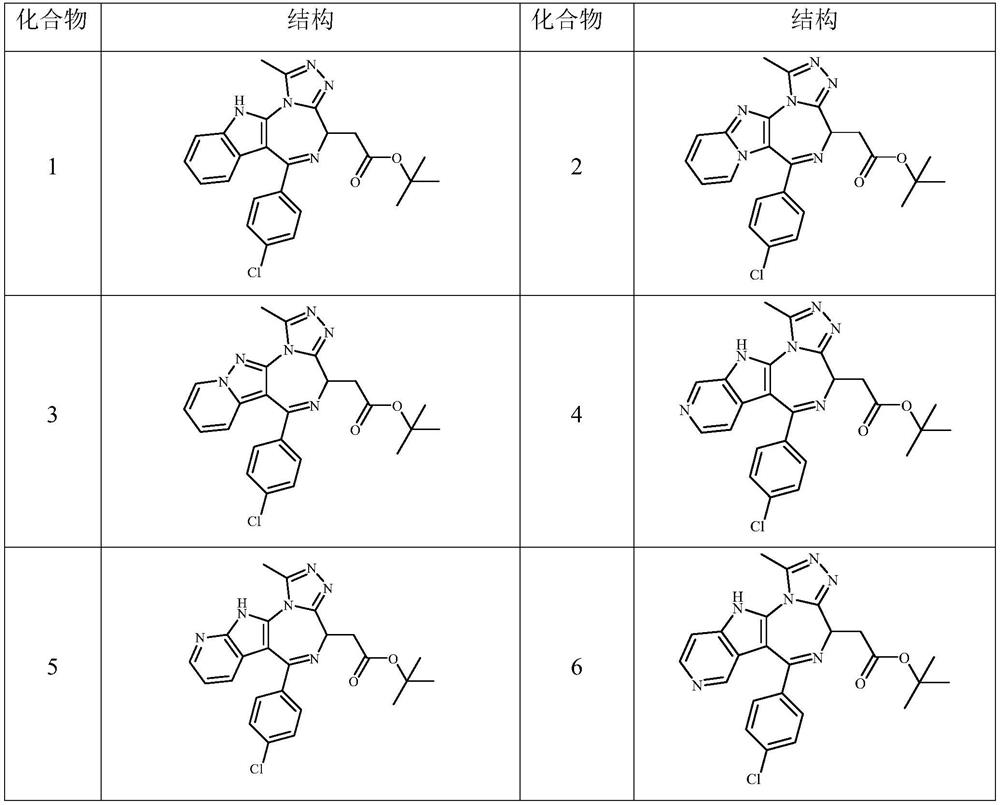
Embodiment 1
[0112] Example 1 ((S)-6-(4-chlorophenyl)-1-methyl-4,11-dihydro-[1,2,4]-triazol[3',4':3,4 ][1, 4] Preparation of tert-butyl acetate (compound 1)-diaza[5,6-b]-4-indolyl)methyl)acetate
[0113] Step 1: Synthesis of 4-Bromo-5-nitro-1H-indole (Intermediate B)
[0114] Add raw material A (81 g, 0.5 mol) into 500 ml of hydrobromic acid aqueous solution, reflux overnight, and HPLC monitors that the reaction is almost complete. Cool down, adjust the pH to 7 with solid sodium carbonate, extract with ethyl acetate (100ml 3 times), and dry the organic phase to obtain 75g of intermediate B as a brown solid with a yield of 62.5%.
[0115] Step 2: Synthesis of (4-chloromethyl)-(5-nitro-1H-indole-4-)-methylketone (Intermediate D)
[0116] Dissolve intermediate B (70g, 0.29mol) in 500mL of tetrahydrofuran, cool down to -78°C under nitrogen protection, add n-butyllithium (2.5M, 140ml, 0.35mol) dropwise, raise the temperature to -40°C for 1 hour, and cool down again To -78°C, a tetrahydrof...
Embodiment 2
[0127] Example 2 ((S)-6-(4-chlorophenyl)-1-methyl-4,11-dihydro-[1,2,4]-triazol[3',4':3,4 ][1, Preparation of tert-butyl 4]-diaza-[5,6-b]-4-[1,8a-dihydro-imidazo[1,2-a]pyridine)acetate (compound 2)
[0128] The preparation method of reference example 1.
[0129] Molecular formula: C 24 h 23 N 6 ClO 2 Molecular weight: 462.16LC-MS(M+H) + :463
[0130] 1H NMR(DMSO)δ1.41(9H,s),δ2.37(3H,s),δ2.92(2H,d),δ4.89(1H,t),δ7.12(2H,d)7.30 (2H,s), δ7.45(2H,d), δ7.90(2H,d)
[0131] Example 2 ((S)-6-(4-chlorophenyl)-1-methyl-4,11-dihydro-[1,2,4]-triazol[3',4':3,4 ][1, Preparation of tert-butyl 4]-diaza-[5,6-b]-4-[1,3a-dihydro-pyrrole[1,5-a]pyridine)acetate (Compound 3)
[0132] The preparation method of reference example 1.
[0133] Molecular formula: C 24 h 23 N 6 ClO 2 Molecular weight: 462.16LC-MS(M+H) + :463
[0134] 1H NMR(DMSO)δ1.41(9H,s),δ2.37(3H,s),δ2.92(2H,d),δ5.01(1H,t),δ7.15(2H,d)7.33 (2H,s), δ7.45(2H,d), δ7.60(1H,t), δ7.90(1H,d)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com