Pharmaceutical composition for preventing cccdna formation of hepatitis b virus
A technology of hepatitis B virus and its composition, which is applied in the fields of compound screening, biological testing, and biological material analysis, and can solve problems such as blocking HBV replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
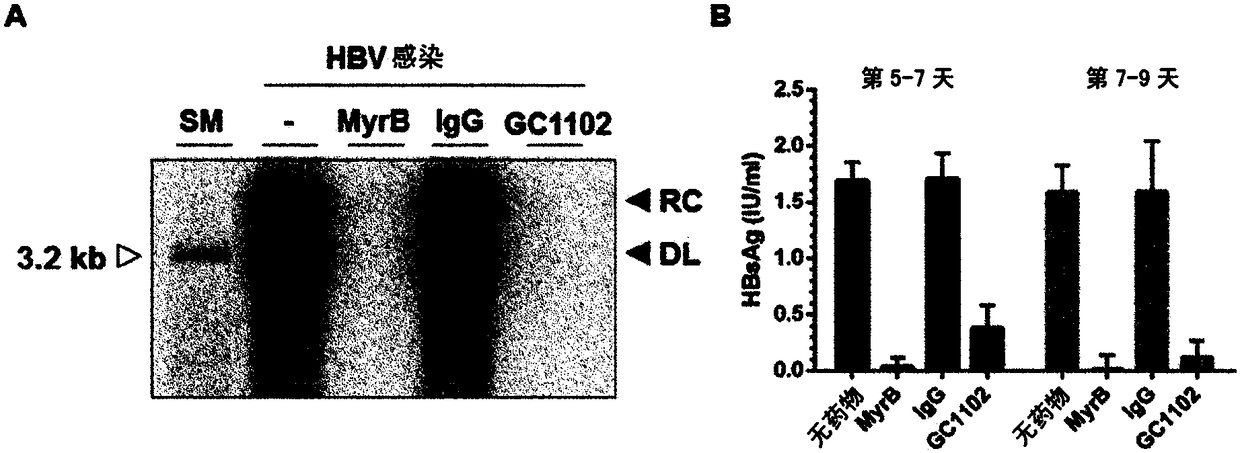
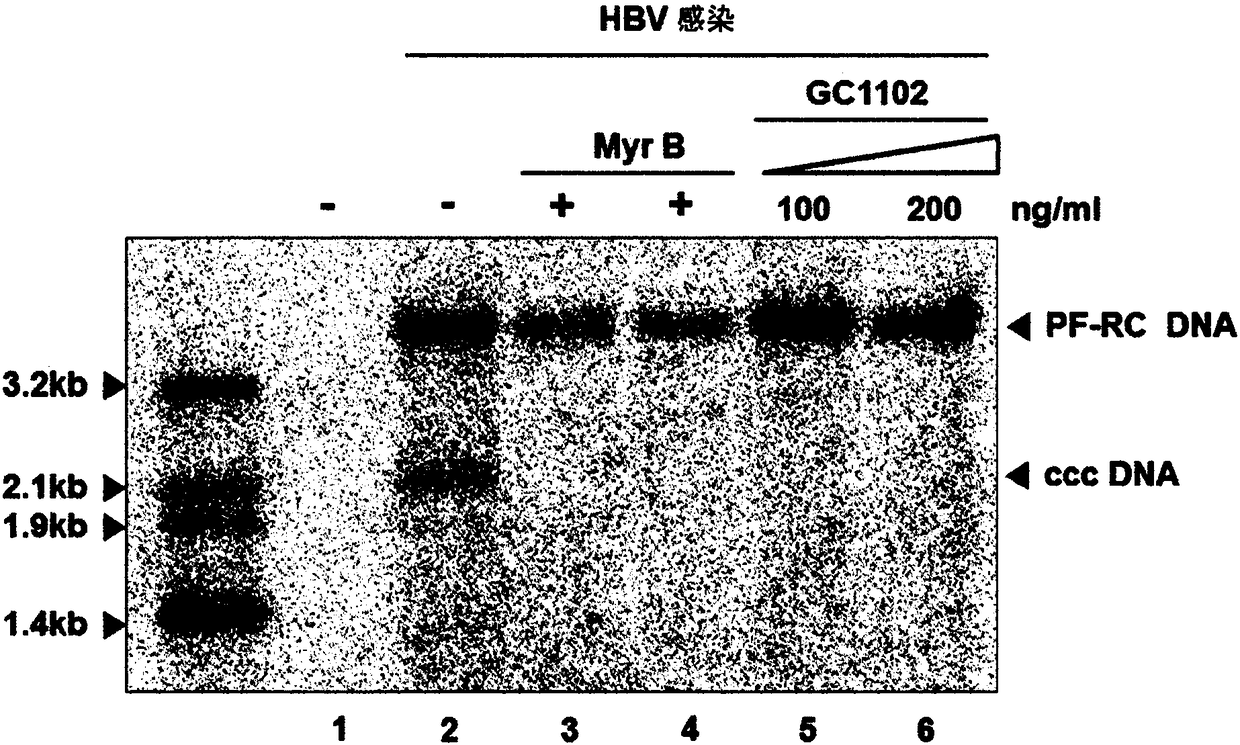
[0083] Example: Estimates of the HBV Infection Inhibitory Efficacy of the Human HBV Antibody (GC 1102) against HBsAg Evaluate
[0084]
[0085] 1) Anti-HBsAg antibody: The antibody (GC1102) used in this experiment is a human antibody represented by the following: the amino acid sequence SEQ ID NO: 1 (HBAb-H4 ); and the amino acid sequence SEQ ID NO: 2 (HBAb-L9) encoding the variable region of the human antibody L chain against the surface antigen of HBV.
[0086] 2) Control group: Both a sample treated with IgG as a negative control and a sample treated with Myrcludex B (MyrB) as a positive control in conducting the experiment were prepared so as to minimize the occurrence of experimental errors. MyrB used as a positive control is a lipopeptide consisting of 47 amino acids in the HBsAg Pre-S1 domain and is now in clinical trials as an HBV entry inhibitor.
[0087]3) Cell culture: The HepG2-NTCP cell line used in this experiment is a cell line stably expressing human NTCP ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com