Sialylated di-Lewis alpha expressed on glycoproteins instead of glycolipids and antibodies thereof as functional cancer targets
A sialylation, glycoprotein technology, applied in the direction of anti-animal/human immunoglobulin, antibody, immunoglobulin, etc., can solve problems such as unacceptable normal distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0184] Generation and preliminary characterization of FG129mAb
[0185] FG129 was produced by immunizing Balb / c mice at 2-month intervals with plasma membrane lipid extracts of LS180 cells (colorectal cell line) incorporated into liposomes using α - Galactosylceramide as adjuvant in the first, third and fourth immunizations, anti-CD40 mAb as adjuvant during the second immunization.
[0186] Analysis of antibody responses to immunization: Antibody titers were first monitored by lipid enzyme-linked immunosorbent assay (ELISA). Flow cytometric analysis (FACS) was performed using LS180 tumor cells and Western blot using LS180. Mice considered to have the best response were boosted intravenously (i.v.) with LS180 plasma membrane lipid extract before fusion compared to prebleed serum controls. Eight days after fusion, supernatants were collected and screened for fresh LS180 tumor cells by flow cytometry. Hybridomas demonstrating cell surface binding using indirect immunofluoresce...
Embodiment 2
[0188] Chimerism of FG129
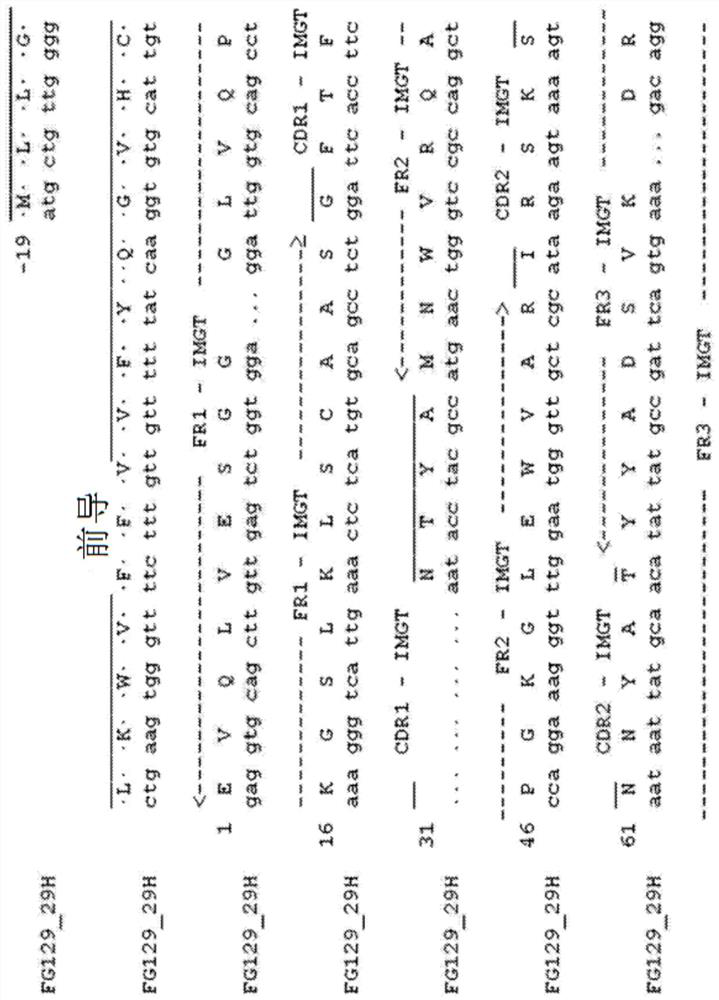
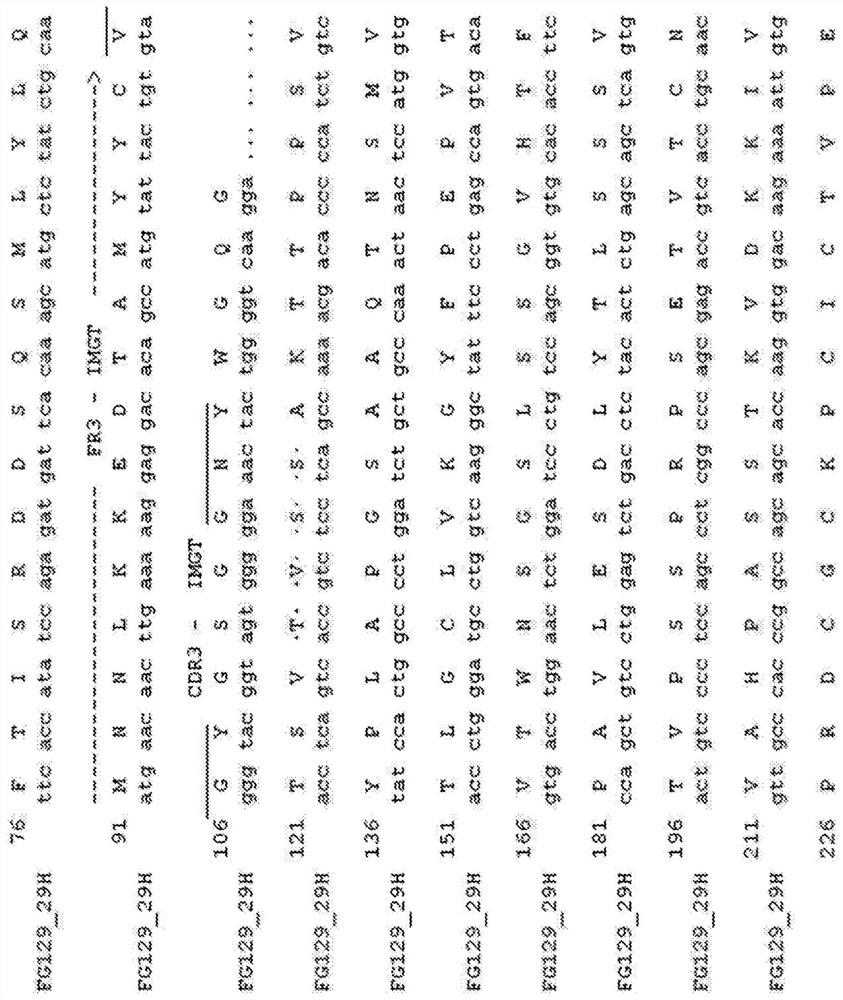
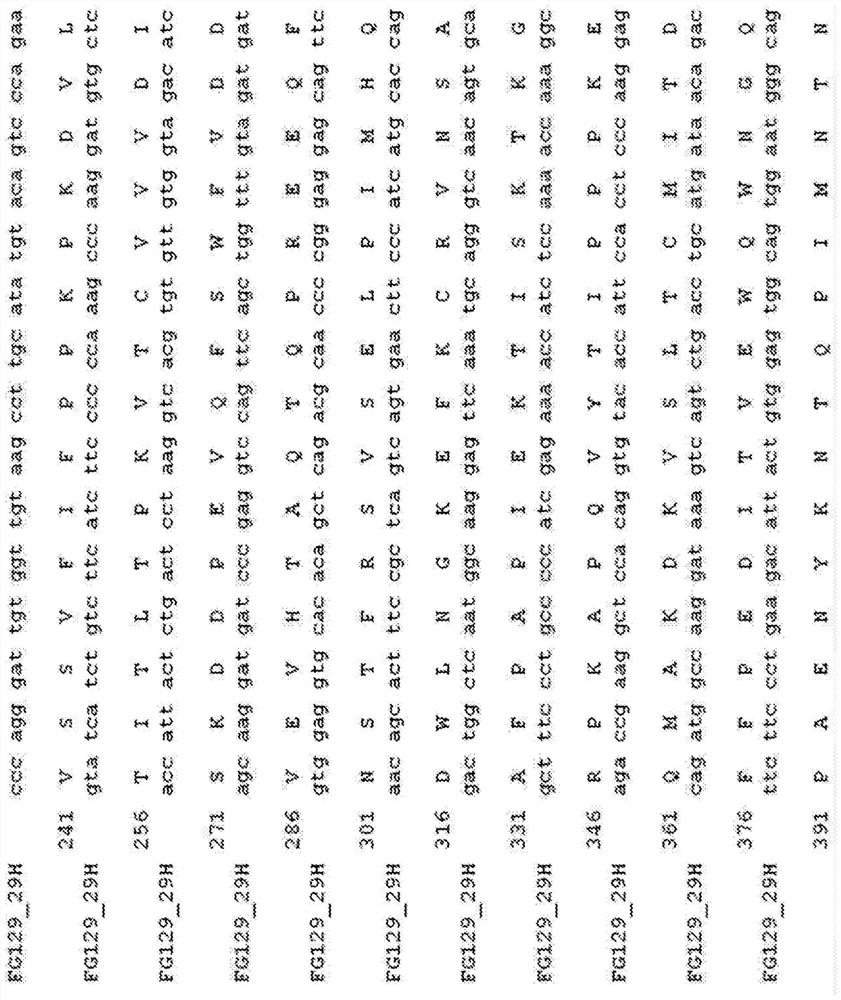
[0189] The term "chimeric antibody" is intended to mean an antibody in which the variable region sequences are derived from one species and the constant region sequences are derived from another species, for example, in which the variable region sequences are derived from a mouse antibody and the constant region sequences are derived from a human antibody. Antibody. Chimeric (or humanized) antibodies of the invention can be prepared based on the sequence of the murine mAb prepared as described above. The heavy chain of FG129mAb is shown in Fig. 1 ( Figure 1a ) and light chain ( Figure 1b The amino acid and nucleotide sequences of the variable and constant regions of ). Numbers refer to the standardized IMGT system used for numbering antibody sequences [49]. CDR1, CDR2 and CDR3 regions are indicated. The FG129 heavy chain belongs to the mouse heavy chain family IGHV10-1*02 (IGHD1-1*01, IGHJ4*01) and has three mutations compared to the parental ...
Embodiment 3
[0192] Definition of epitopes recognized by FG129 and CH129 mAbs
[0193] MAb FG129 is a mouse IgGlk isotype produced by immunizing Balb / c mice with glycolipid extracts from the colorectal cell line LS180. Glycan profiling of ≥600 natural and synthetic glycans by CFG shows that FG129 binds sialylated di-Lewis a (100%) and sialylated Lewis a-x High specificity (89%). It can also be associated with monosialylated Lewis a (89%) bound, but only on the long vector (sp8) but not on the short vector (sp0), indicating that at least 4 carbohydrates or enough space is required to allow insertion of three carbohydrate residues for proper binding Conformation exists in the antibody sequence ( Figure 3a ).
[0194] To analyze whether these glycans are expressed on glycoproteins or glycolipids from tumor cell lines, FG129 binding was assessed by Western blotting ( Figure 3b ). Tumor lysates or tumor glycolipid extracts from colorectal (Colo205HCT-15 and LS180) and pancreatic cell l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com