Development of novel automated screening method for detection of FVIII inhibitors
A technology for inhibitors and coagulation agents, applied in the field of identification of coagulation inhibitors in patient samples, which can solve the problems of missing inhibitor tests and increased reporting errors in inhibitor assessment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
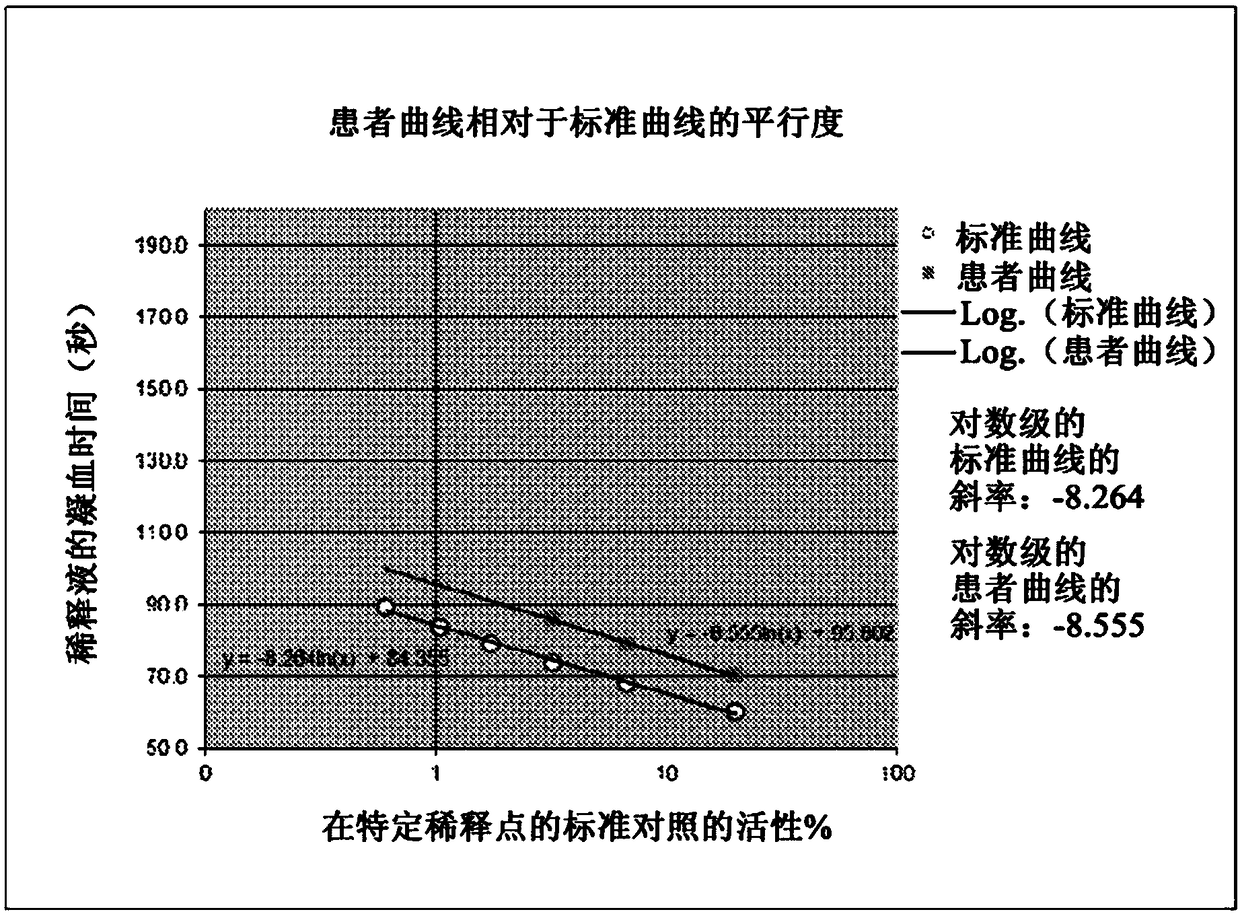
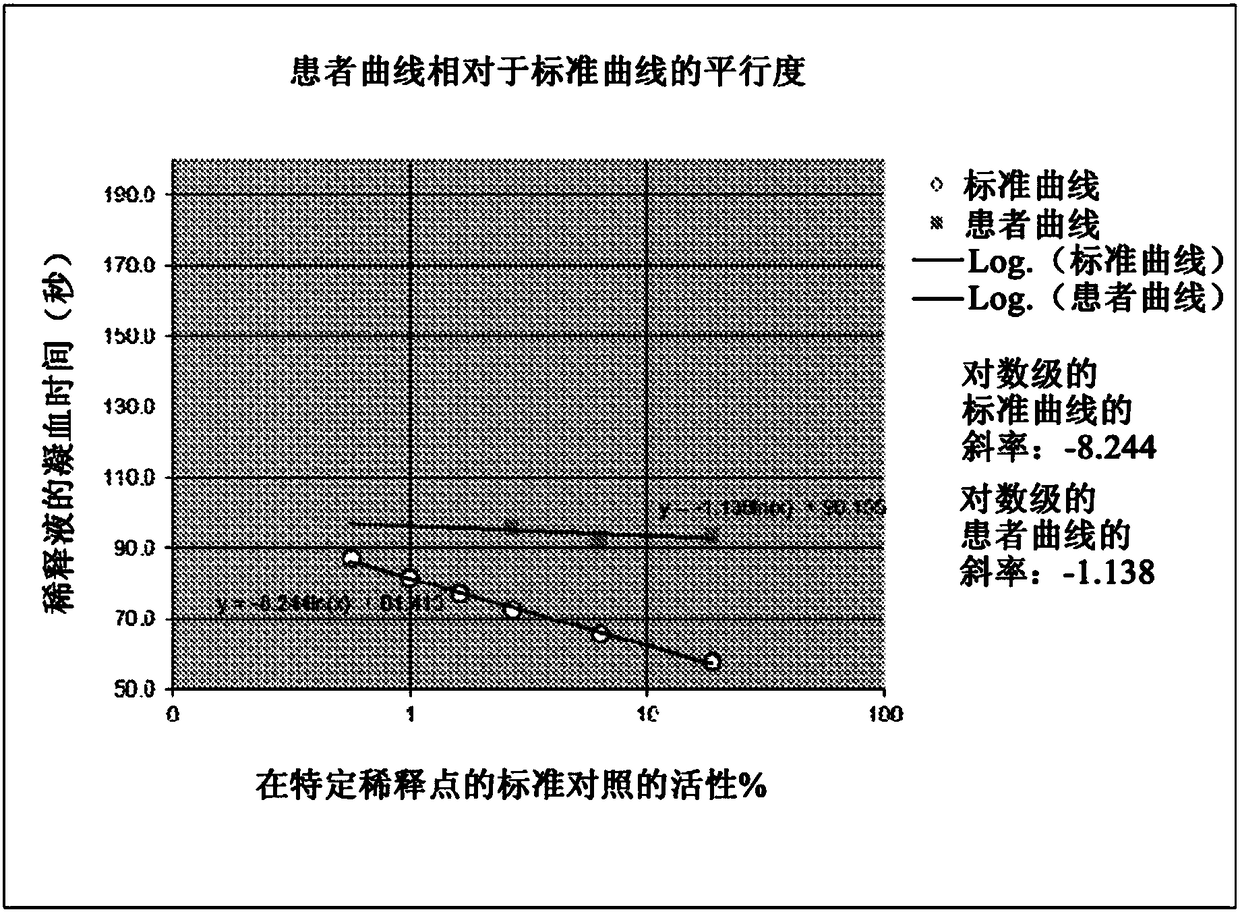
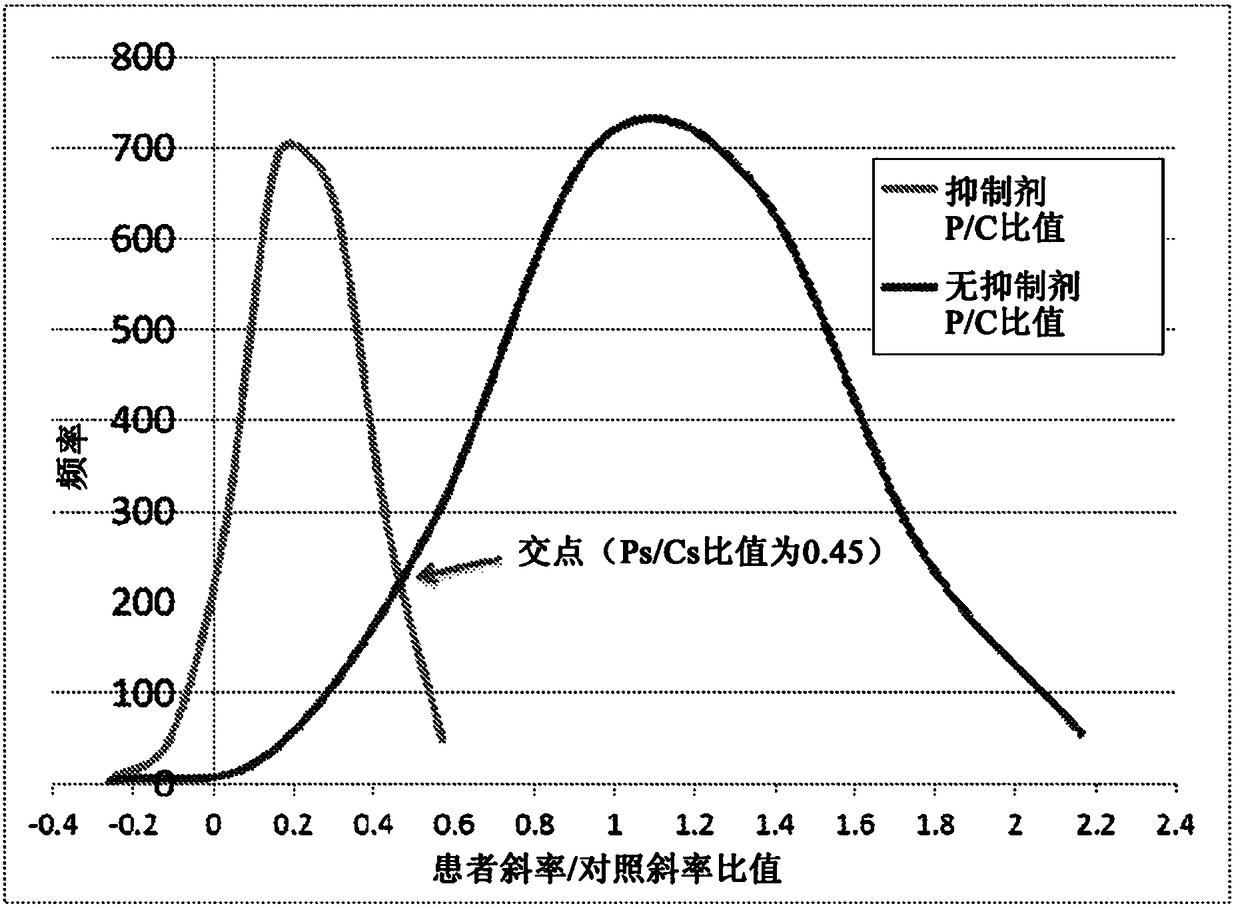
[0044] As noted above, a typical Factor VIII assay determines Factor VIII activity (in percent) from a standard curve. The standard curve was generated by plotting the measured clotting time (in seconds) versus the known standard percent activity at several specific dilution points. Factor VIII activity in patient samples was then determined by mixing dilutions of patient plasma with equal amounts of known factor-deficient plasma, then plotting the clotting times observed for specific patient sample dilutions to obtain a standard curve, and from this standard The curve calculates whether the factor is present in the patient's plasma. To minimize the possibility of underreporting activity levels or missing the presence of inhibitors, when plotted logarithmically, the line given by the clotting time of the patient's plasma dilution should be parallel to that given by the systemic control plasma dilution. out the line.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com