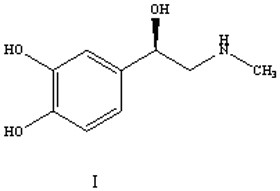
A kind of preparation method of (r)-epinephrine
A technology of epinephrine and compound, applied in the field of preparation of (R)-epinephrine, to achieve the effects of high optical purity, mild reaction conditions and high optical yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
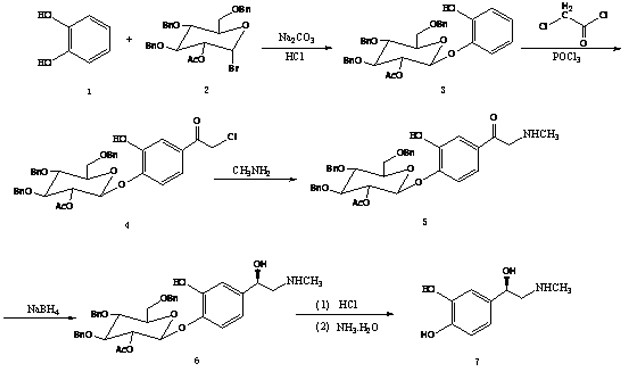
[0024] Such as figure 2 Shown, the preparation method of (R)-epinephrine of the present invention comprises the following steps:
[0025] 1) Add 11.0g (1mol) of catechol (compound 1) and 60.9g (1.1mol) of compound 2 into the reaction flask, then add 50mL of tetrahydrofuran and 21.2g of 50% aqueous sodium carbonate solution, and stir for 5 hours at room temperature Add 1N hydrochloric acid to the reaction solution to adjust the pH of the solution to 6-6.5, evaporate the tetrahydrofuran under reduced pressure, extract the residue with dichloromethane, 50 mL each time, extract three times, combine the dichloromethane layers, and wash with 50 mL of saturated sodium chloride aqueous solution Once, dried over anhydrous sodium sulfate, filtered and evaporated to dryness to obtain 52.6 g of compound 3 with a yield of 90%.
[0026] 2) Put 52.6g (0.09mol) of compound 3, 10.2g (0.09mol) of chloroacetyl chloride and 6.9g (0.045mol) of phosphorus oxychloride into the reaction flask, rais...
Embodiment 2
[0031] The preparation method of (R)-epinephrine of the present invention comprises the following steps:
[0032] 1) Add catechol (compound 1) 11.0g (1mol) and compound 2 60.9g (1.1mol) into the reaction flask, then add acetonitrile 50mL and 50% sodium carbonate aqueous solution 21.2g, stir and react at room temperature for 5 hours Add 1N hydrochloric acid to the reaction solution to adjust the pH of the solution to 6-6.5, evaporate acetonitrile under reduced pressure, and extract the residue with dichloromethane, 50 mL each time, three times, combine the dichloromethane layers, and wash with 50 mL of saturated sodium chloride aqueous solution Once, dried over anhydrous sodium sulfate, filtered and evaporated to dryness to obtain 352.3 g of the compound with a yield of 89.4%.
[0033] 2) Put 52.3g (0.09mol) of compound 3, 10.2g (0.09mol) of chloroacetyl chloride and 6.9g (0.045mol) of phosphorus oxychloride into the reaction flask, raise the temperature to 60±2°C for 3.5 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com