New compounds inhibitors of the YAP/TAZ-TEAD interaction and their use in the treatment of malignant mesothelioma
一种化合物、O-R11的技术,应用在新化合物抑制剂领域,能够解决未报道抑制活性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0106] Such dosage forms, excipients and methods for their preparation are well known in the art, for example in (Handbook of pharmaceutical Excipients, Rowe et al., Seventh Edition, June 2012; Rules and Guidance For Pharma Manufacturers and distributors 205, Medecines and Healthcare products Regulatory Agency, UK, London).
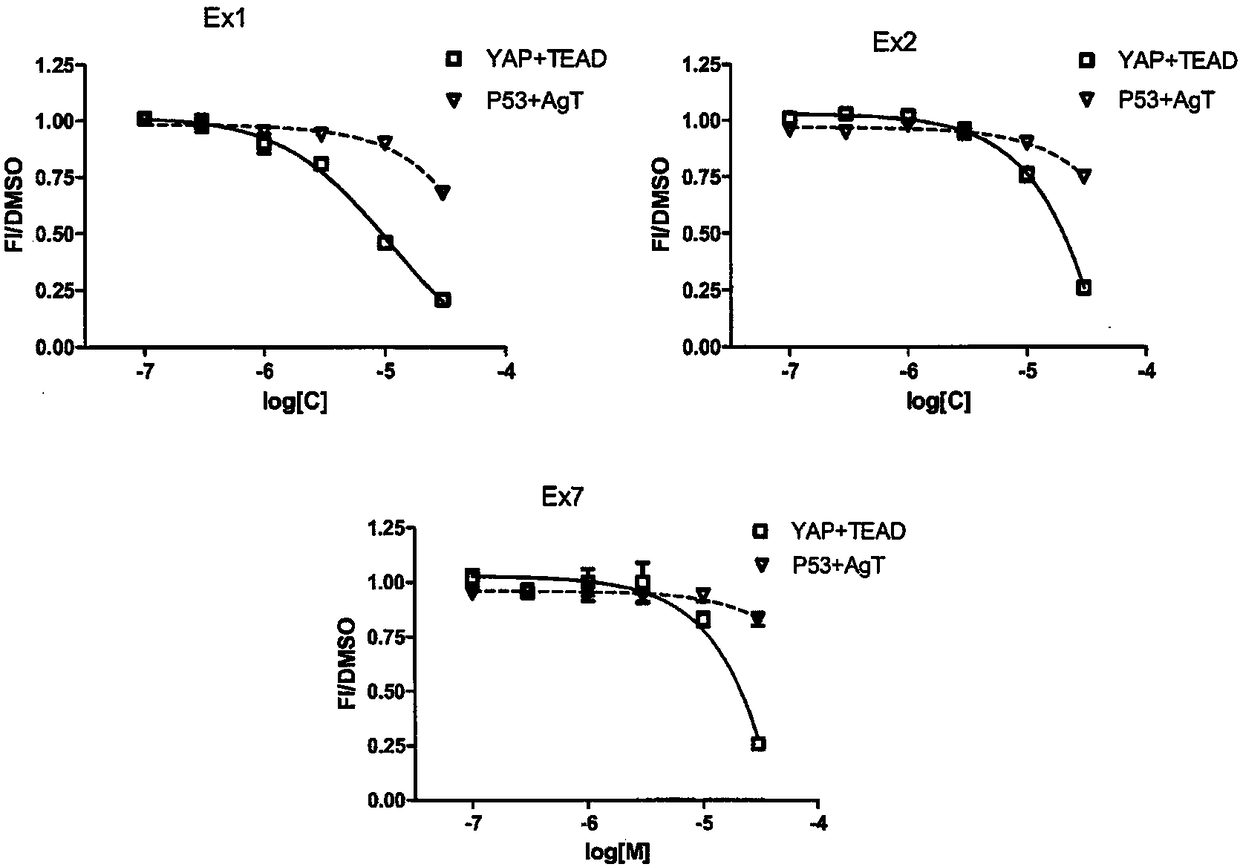
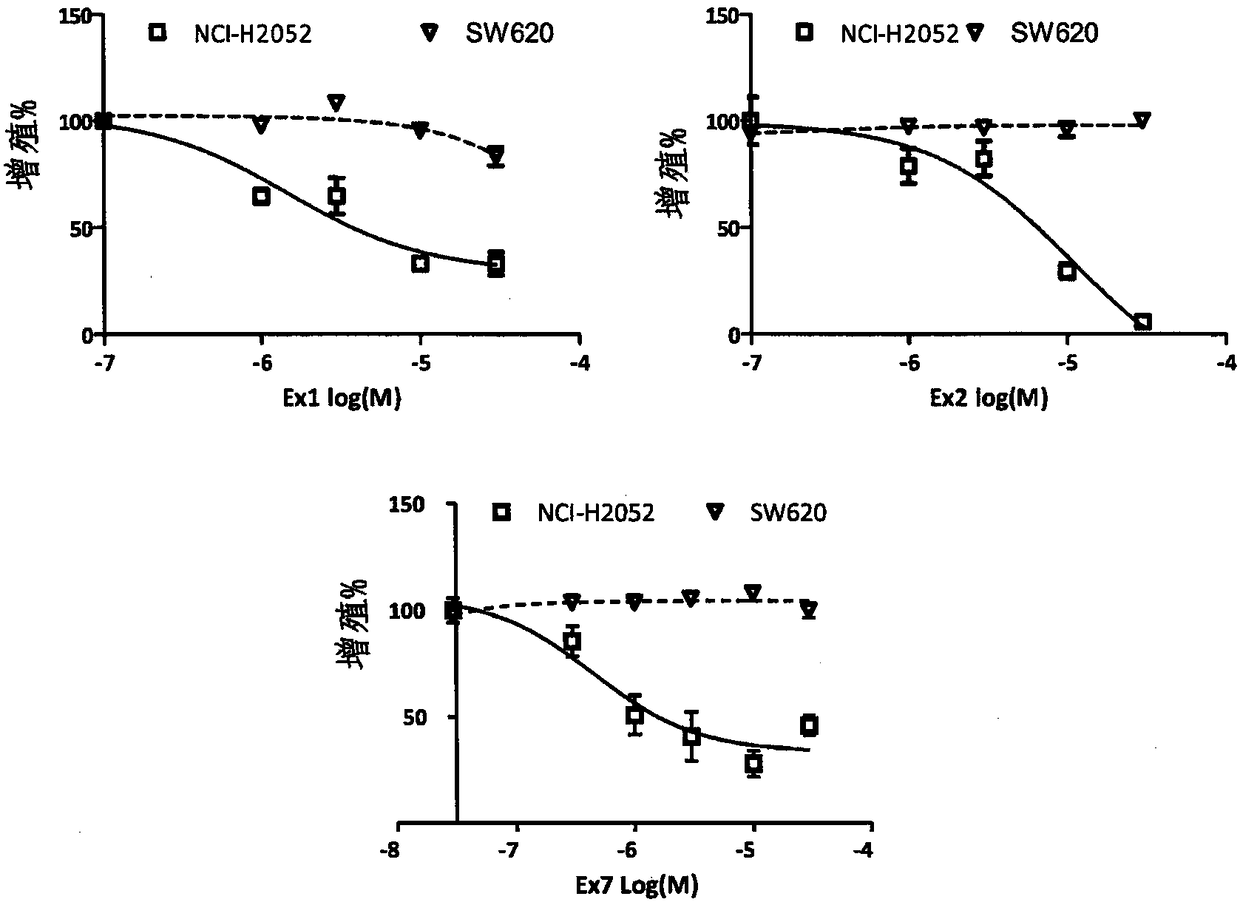
[0107] Accordingly, the present invention also relates to inhibitors of the YAP / TAZ-TEAD interaction, and the use thereof of compounds of formula (I) as described herein, for therapy; in particular for the therapy in which YAP is localized in the nucleus of tumor cells For any cancer indication (such as lung cancer, thyroid cancer, ovarian cancer, colorectal cancer, prostate cancer, pancreatic cancer, esophageal cancer, liver cancer, breast cancer and skin cancer); more particularly for the treatment of malignant mesothelioma use.
[0108] The present invention also relates to a method for the treatment of cancer in a patient in need thereof, comprising ...
Embodiment 1
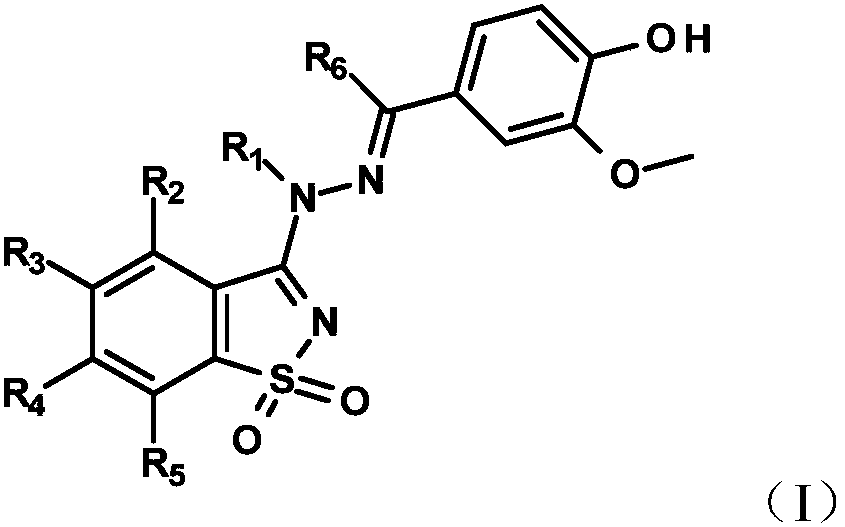
[0208] Example 1: 4-[(E)-[(1,1-dioxo-1,2-benzothiazol-3-yl)-methyl-hydrazono]methyl]-2-methoxy -phenol
[0209] Step A: 3-Chloro-1,2-benzothiazole-1,1-dioxide
[0210]
[0211] A mixture of saccharin (4.00 g, 21.84 mmol, 1 equiv), thionyl chloride (2.38 mL, 32.75 mmol, 1.5 equiv) and a catalytic amount of DMF (120 μL) in 1,4-dioxane (20 mL) Heated at reflux for 24h. The reaction mixture was concentrated and the residue was recrystallized from toluene, filtered, washed with cold toluene and dried under vacuum at 50 °C to give a crushed white solid. The filtrate was concentrated under reduced pressure and recrystallized from toluene, filtered and washed with cold toluene. The combined solids were washed by cold toluene and dried under vacuum at 50 °C to yield 3-chloro-1,2-benzothiazole-1,1-dioxide (3.28 g, 74%). 1 H NMR (DMSO-d 6 , 300MHz): δ8.19 (dd, J = 1Hz, J = 7Hz, 1H); 7.99 (m, 3H).
[0212] Step B: 2-Methoxy-[4-(E)-(methylhydrazono)methyl]phenol
[0213]
[02...
Embodiment 2
[0218] Example 2: 4-[(E)-[(1,1-dioxo-1,2-benzothiazol-3-yl)-(2-hydroxyethyl)-hydrazono]methyl]- 2-methoxy-phenol
[0219] Step A: 4[(E)-(2-Hydroxyethylhydrazono)methyl]-2-methoxy-phenol
[0220]
[0221] Starting with 4-hydroxy-3-methoxybenzaldehyde (1.00 g, 6.57 mmol, 1 equiv) and 2-hydroxyethylhydrazine (446 μL, 6.57 mmol, 1 equiv), using the same method as detailed in Example 1, Step B The compound was prepared by the same procedure as above to give 4-[(E)-(2-hydroxyethylhydrazono)methyl]-2-methoxy-phenol (1.84 g, 89%) as a yellow solid. 1 H NMR (DMSO-d 6 , 300MHz): δ7.54(s, 1H); 7.08(d, J=1.8Hz, 1H); 6.82(dd, J=1.8Hz, J=8.4Hz, 1H); 6.74(d, J=8.4Hz ,1H); 3.79(s,3H); 3.54(t,J=6Hz,2H); 3.13(m,2H).
[0222] Step B: 4-[(E)-[(1,1-dioxo-1,2-benzothiazol-3-yl)-(2-hydroxyethyl)hydrazono]methyl]-2- Methoxy-phenol
[0223]
[0224] From 4-[(E)-(2-hydroxyethylhydrazono)methyl]-2-methoxy-phenol (800.0 mg, 3.81 mmol, 1.1 equivalents) and 3-chloro-1,2-benzo Starting with thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com