Metal oxide supported noble metal catalyst, preparation method and use
A technology of noble metal catalysts and oxides, applied in metal/metal oxide/metal hydroxide catalysts, catalyst activation/preparation, physical/chemical process catalysts, etc. Problems such as the interaction between noble metals and supports, and the lack of good stability of the catalyst, etc., to achieve good methane low-temperature oxidation activity, good industrial application prospects, and uniform morphology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
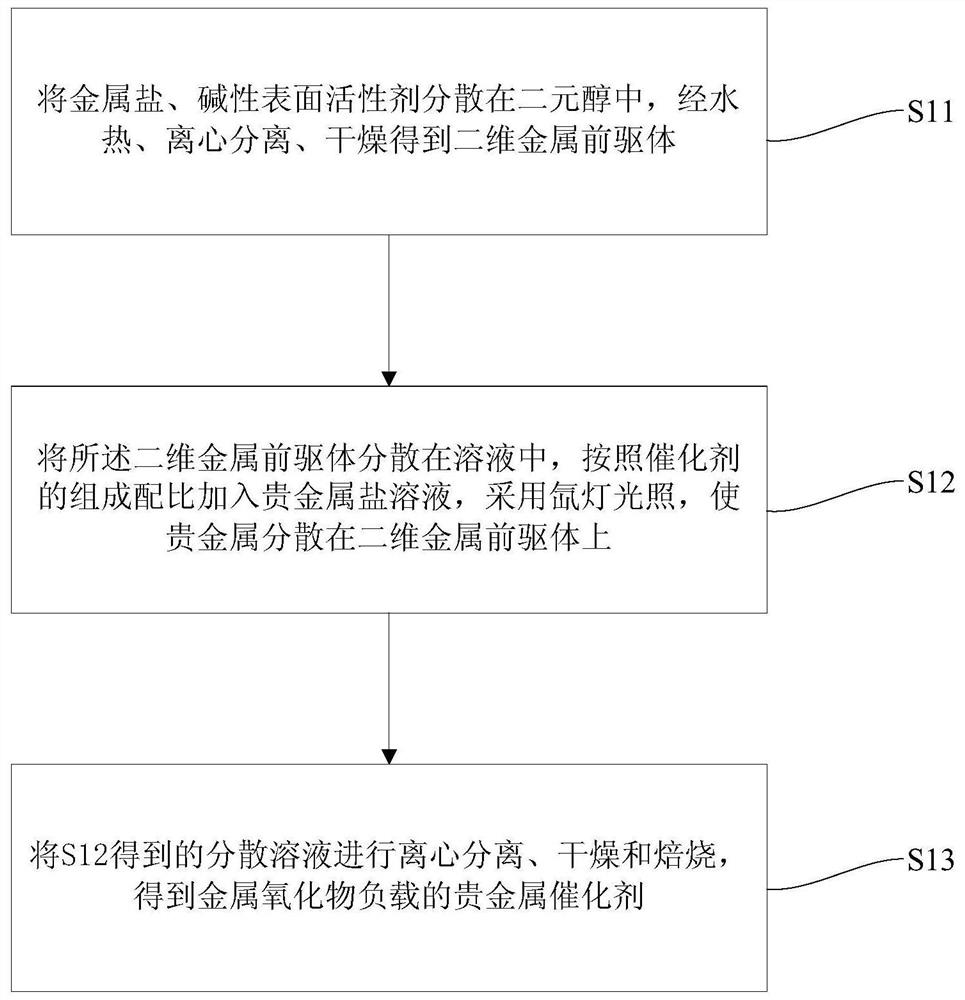
[0046] Such as figure 1 As shown, the preparation method of the metal oxide-supported noble metal catalyst of the present embodiment comprises the following steps:
[0047] Such as figure 1 As shown, step 1) S11 is firstly carried out, dispersing the metal salt and alkaline surfactant in the dihydric alcohol, and obtaining a two-dimensional metal precursor by hydrothermal, centrifugal separation, and drying.
[0048] As an example, the ratio of the metal salt, alkaline surfactant and glycol is 0.5-10 mmol: 0.5-5 mmol: 75 mL. For example, the ratio of metal salt and alkaline surfactant can be 0.5~1mol: 0.5~5mmol, 1~5mol: 0.5~5mmol, 5~10mmol: 0.5~5mmol; the ratio of metal salt and glycol can be 0.5~1mmol : 75mL, 1-5mmol: 75mL or 5-10mmol: 75mL, etc. A further preferred ratio of metal salt, alkaline surfactant and dihydric alcohol is 7.5mmol: 1.3mmol: 75mL.
[0049] As an example, dibasic alcohol is used as a solvent and a chelating agent in the reaction, and is selected from...
Embodiment 1
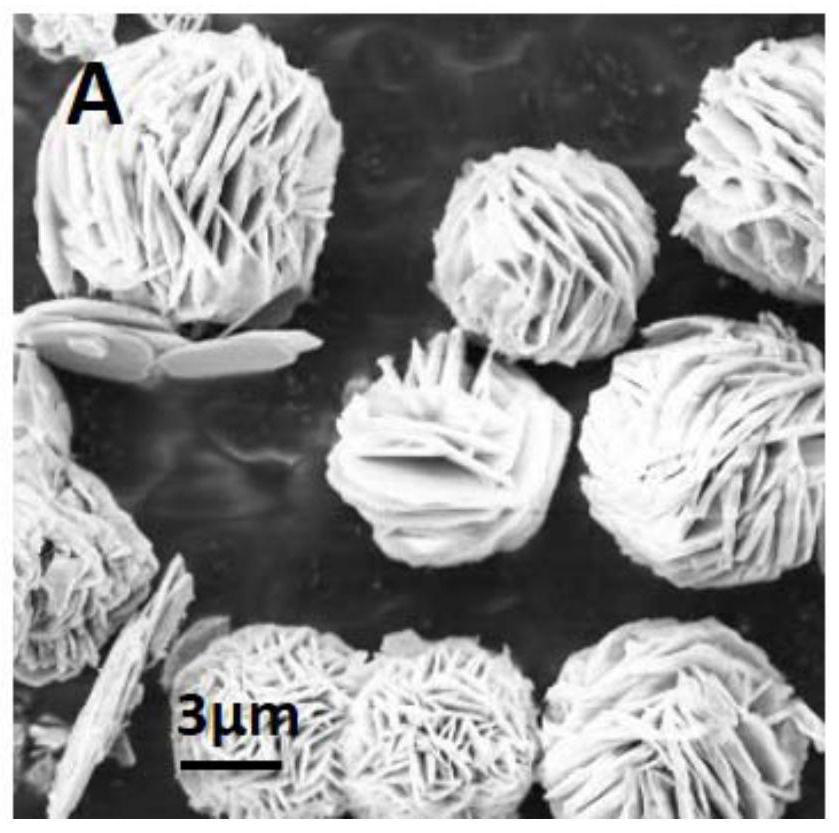
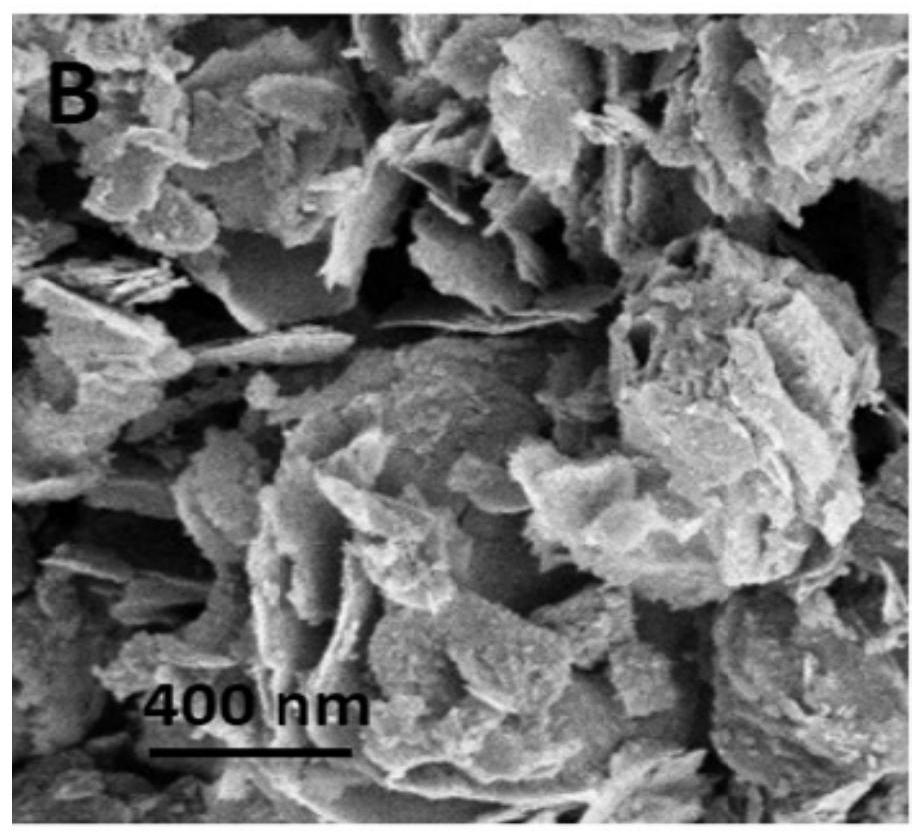
[0062] This embodiment provides a 3%Pd-Co 3 o 4 The preparation method of nanosheets (3% refers to the ratio of the quality of noble metal elements to the total mass of noble metal elements and metal oxides, the following examples are defined the same), comprising the steps of: weighing 7.5mmol cobalt acetate tetrahydrate (Co(CH 3 COO) 2 4H 2 O) and 1.3mmol PVP (Mw=24000) were dissolved in 75mL of ethylene glycol, stirred and dissolved, transferred to a high-pressure reactor, heated in water at 180°C for 12h, cooled to room temperature, centrifuged and washed with deionized water, Vacuum dried at 60°C for 12 hours to obtain hydroxyethyl cobalt nanosheets, see SEM image Figure 2a . Hydroxyethyl cobalt nanosheets were dispersed in water to obtain a dispersion of hydroxyethyl cobalt (39mM, 30mL), and 5mL of 8.8mg Pd(NO 3 ) 2 solution, after which the dispersion system was placed in a xenon lamp (6A, the light intensity density at 365nm was 50mW / cm 2 ) at room temperature ...
Embodiment 2
[0064] This embodiment provides a 1% Pd-Co 3 o 4 The preparation method of nano sheet, comprises the steps: take by weighing 10mmol cobalt acetate tetrahydrate (Co(CH 3 COO) 2 4H 2 O) and 2mmol PVP (Mw=48000) were dissolved in 75mL of ethylene glycol, stirred and dissolved, transferred to an autoclave, heated in water at 180°C for 12h, cooled to room temperature, centrifuged and washed with deionized water, and placed in Vacuum drying at 60° C. for 12 h to obtain hydroxyethyl cobalt nanosheets. Hydroxyethyl cobalt nanosheets were dispersed in water to obtain a dispersion of hydroxyethyl cobalt (39mM, 30mL), and 5mL of 2.9mg Pd(NO 3 ) 2 solution, after which the dispersion system was placed in a xenon lamp (10A, the light intensity density at 365nm was 100mW / cm 2 ) at room temperature for 5 min. The Pd-hydroxyethyl cobalt obtained by the reaction was centrifuged, dried, and roasted at 350°C for 5 hours to obtain 1% Pd-Co 3 o 4 Nanosheets. 1%Pd-Co 3 o 4 The nanosheet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com