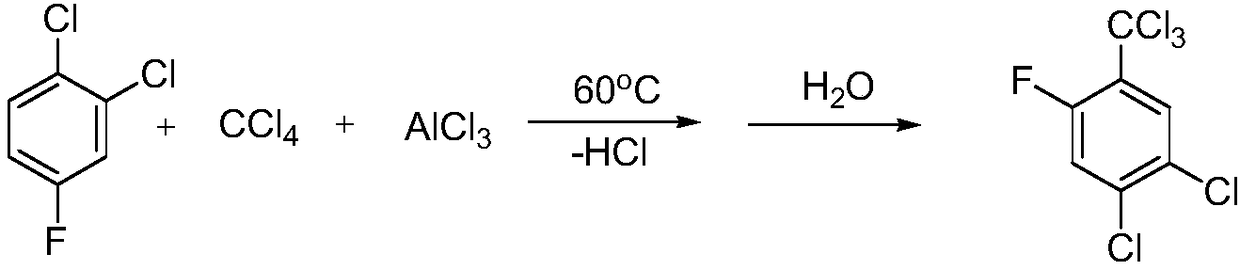
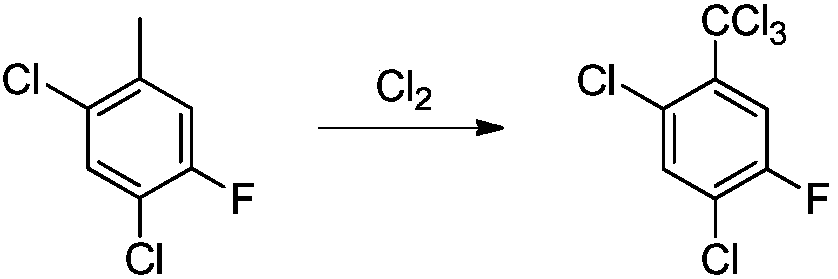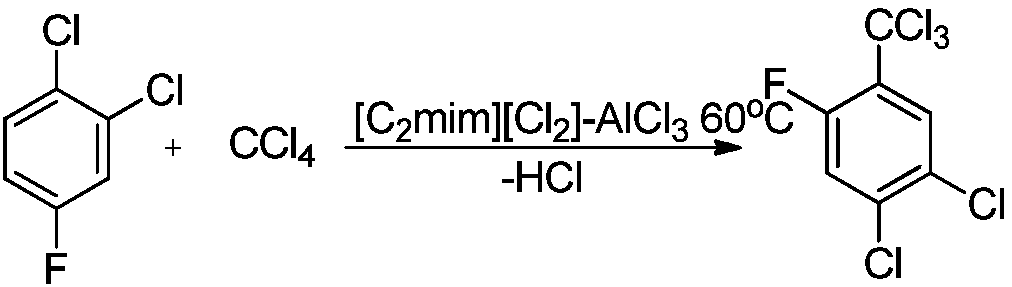Synthesis method of 2,4-dichloro-5-fluorin(trichloromethyl)benzene
A technology of trichloromethyl and synthetic methods, applied in the field of chemical technology, can solve the problem of many reaction by-products, achieve the effects of convenient recycling, lower reaction temperature, and higher reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0024] 35.2 g of bis(1-ethyl-3-methylimidazolium chloride) and 52.3 g of AlCl 3 Place in a nitrogen-protected reaction vessel and stir at normal temperature to obtain 87.5g of 1,2-bis(N-methylimidazolium)ethane chloroaluminate ([C 2 mim][Cl 2 ]-AlCl 3 ).
Embodiment 2-1
[0027] Add 16.5 g (0.1 mol) of 2,4-dichlorofluorobenzene, CCl 4 75g (0.4mol), ionic liquid [C 2 mim][Cl 2 ]-AlCl 3 87.5g, heat up to 60°C and keep warm for 4 hours; after the reaction, let stand to separate and separate, recover the upper ionic liquid, and the lower organic phase is distilled under reduced pressure to obtain the target product 2,4-dichloro-5-fluoro-(trichloro Methyl)benzene 24.2g, Mp: 293~294°C, yield 92.9%.
Embodiment 2-2
[0029] Add 16.5 g (0.1 mol) of 2,4-dichlorofluorobenzene, CCl 4 75g (0.4mol), the ionic liquid [C that embodiment 2-1 reclaims 2 mim][Cl 2 ]-AlCl 3 87.5g, heat up to 60°C and keep warm for 3 hours; after the reaction is over, let it stand for stratification, recover the upper ionic liquid, and the lower organic phase is distilled under reduced pressure to obtain the target product 2,4-dichloro-5-fluoro-(trichloro Methyl)benzene 23.3g, Mp: 293~294°C, yield 92.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com