Method for establishing mouse model of cardiac volume overload
A model and capacity technology, applied in the field of biomedicine, can solve problems such as reflux, high mortality, and massive bleeding, and achieve convenient operation and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Establishment of cardiac volume overload mouse model
[0056] For mice undergoing aortic valve regurgitation surgery (true surgery), after intraperitoneal anesthesia with ketamine (150 mg / kg) and xylazine (10 mg / kg), the mice were fixed on a heating plate, and the ends of the limbs of the mice were smeared with The conductive paste is then attached to the conductive area of the heating plate. Subsequently, a 27G cannula 2 with a built-in 0.16mm diameter steel wire 1 was inserted through the right common carotid artery (RCCA) ( figure 1 with 2 shown). Under the guidance of high-frequency ultrasound, the root of the aortic valve is clearly displayed, the cannula is advanced to the front of the aortic valve, and the built-in steel wire is stretched out from the cannula to puncture part of the aortic valve ( figure 2 shown). During the modeling process, the Doppler ultrasound of the blood flow at the aortic arch was monitored at any time, and the modeling w...
Embodiment 2
[0057] Embodiment 2 Realization of Quantitative Control of Reflux Degree
[0058] Non-invasive and real-time monitoring of aortic arch blood flow by high-frequency ultrasound of small animals can quantify the degree of reflux, and according to the needs of experiments, mouse models with different degrees of reflux can be produced ( Figure 4 ). This is a huge advantage over previous arteriovenous fistula volume overload models that cannot quantify the severity of stoma after stoma.
Embodiment 3
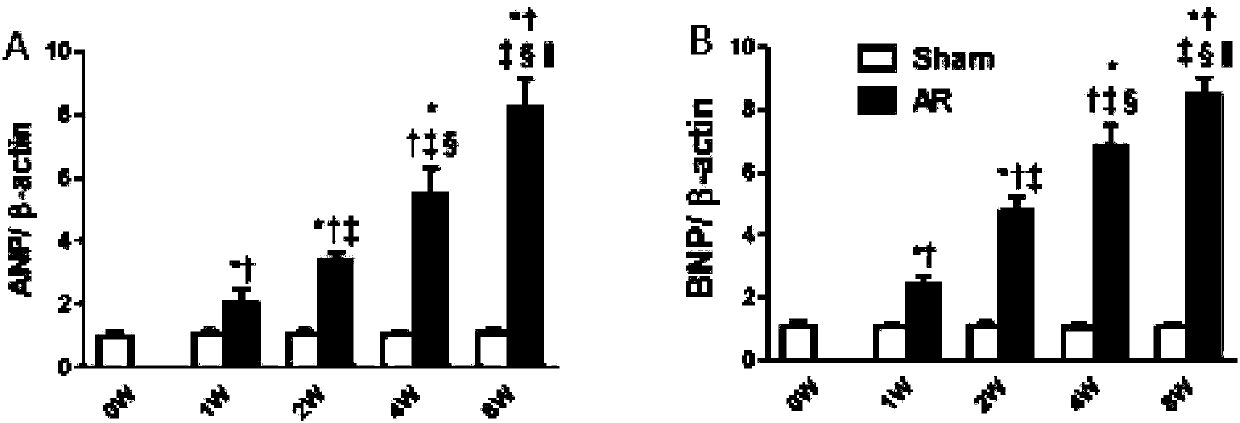
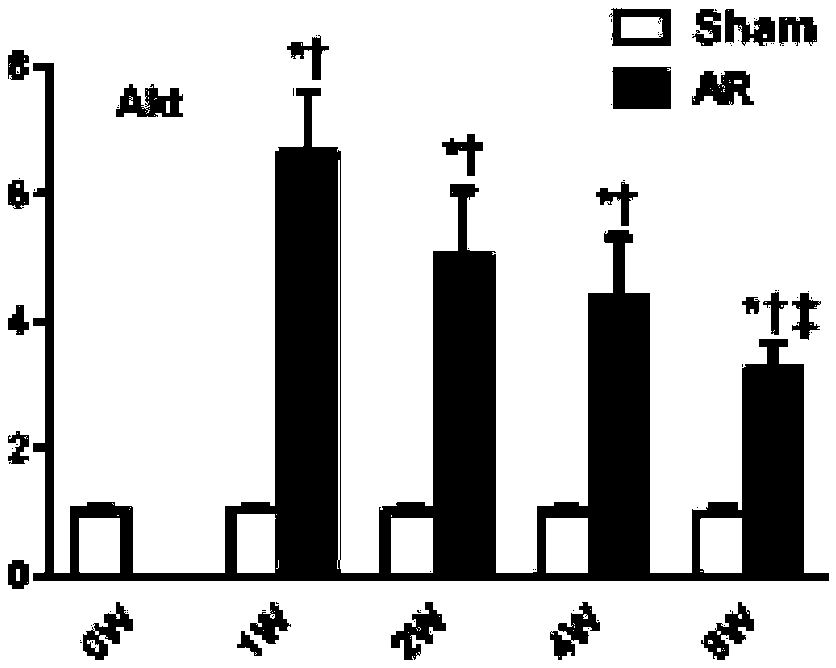
[0059] Example 3 Changes in Left Heart Structure and Cardiac Function of Mice Before and After 1 Week, 2 Weeks, 4 Weeks and 8 Weeks in Moderate Reflux State
[0060] Figure 5 It is shown that the reverse blood flow of the aortic arch of mice at 1 week, 2 weeks, 4 weeks, and 8 weeks after operation is obvious, and there is no obvious change at each time point after operation; Image 6 Shown are the changes of the long-axis M-mode ultrasonography of the mouse left ventricle before operation and 1 week, 2 weeks, 4 weeks, and 8 weeks after operation. It can be seen that the left ventricular diameter is significantly enlarged; Figure 7 Significant increase in left ventricular end-diastolic diameter, Figure 8 Significant increase in left ventricular end-systolic diameter; Figure 9 A slight increase in left ventricular end-diastolic posterior wall thickness two weeks after surgery, Figure 10 The left ventricular end-systolic posterior wall thickness increased slightly two wee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com