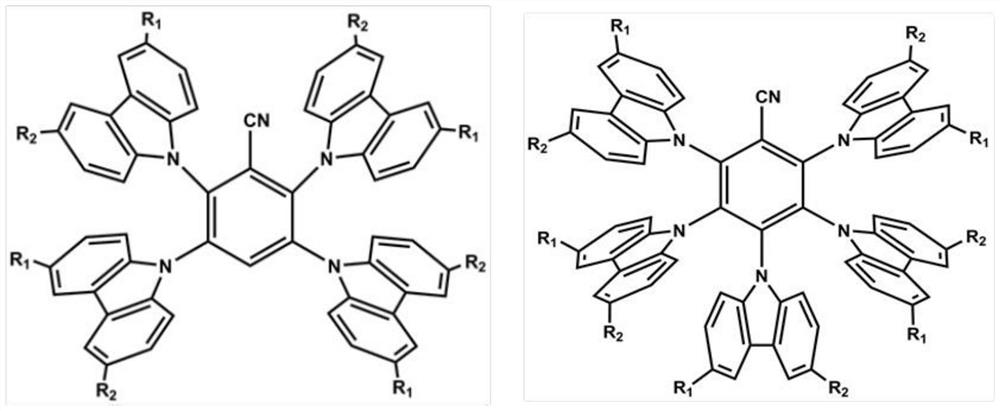
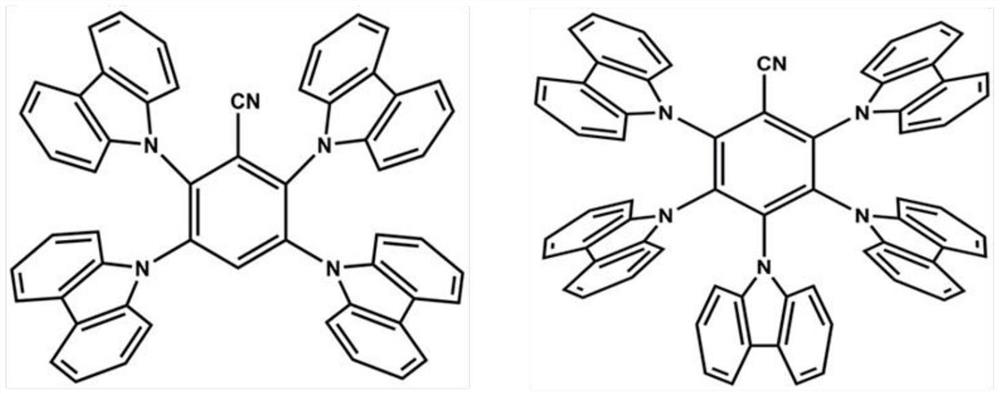
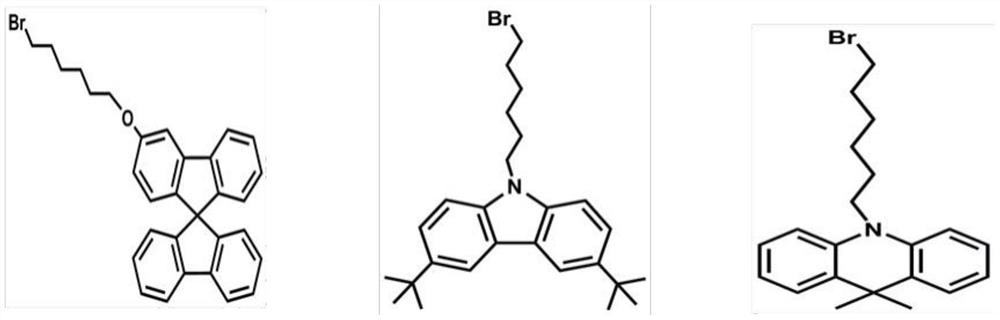
A solution-processable thermally activated delayed fluorescent material and its preparation method
A technology of thermally activated delay and fluorescent materials, which is applied in the fields of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc. It can solve the problems of low luminous brightness and efficiency, slow research progress, long synthesis route, etc., and achieve excellent devices performance, reduced concentration quenching, and improved performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the synthesis of compound C1
[0036] Step 1, S1CH 3 Synthesis of O
[0037] Add 3-bromo-9,9-spirobifluorene (10g, 25.12mmol), sodium methoxide (50mL, 25.12mmol), CuI (15g, 78.94mmol), N,N-dimethylformamide into a 500mL reaction flask (200mL), under nitrogen protection, react at 120°C for 24h. After the reaction, cool to room temperature, add a large amount of water to stir, and filter with suction to obtain a crude product. Then, purified by column chromatography to obtain S1CH 3 O is a white solid, and the yield of S1CH3O is 70%.
[0038] Step 2, Synthesis of S1OH
[0039] Add S1CH to the reaction vial 3 O (6g, 17.19mmol) was dissolved by adding 30mL of dichloromethane solution. Under ice-bath conditions, a solution of 3-boron bromide (15mL) in dichloromethane solution (30mL) was added dropwise with a separatory funnel. The reaction was stirred at 0° C. for 4 h, quenched with methanol (20 mL), and washed with sodium bicarbonate to adjust the pH of...
Embodiment 2
[0050] Embodiment 2: Synthesis of C2
[0051]In the above example, the 3,6-dihydroxy-9-hydrogen-carbazole reacted with S1O-Br was replaced with 3-hydroxycarbazole, and the product C2 was obtained through the same synthesis method as in Example 1. Yield 70%.
[0052] Mass spectrum: 2484.05.
[0053] Elemental analysis, the results are as follows: C: 86.47, H: 5.56, N: 2.82, O: 5.15.
Embodiment 3
[0054] Embodiment 3: Synthesis of C3
[0055] Step 1, Synthesis of Branched CZ-Br
[0056] Add di-tert-butylcarbazole (6.7g, 23.92mmol), 1,6-dibromohexane (33mL, 143.5mmol), KOH (15g, 267.85mmol / dissolve in 10mL water first) in toluene solution (80mL) , tetrabutylammonium bromide (1 g, 1.8 mmol). Under the protection of nitrogen, the reaction was stirred at 80° C. for 3 h, and directly spin-dried after the reaction. Purified by column chromatography to obtain the CZ-Br product with a yield of 71%.
[0057] Step 2, Synthesis of CZ-CZO
[0058] Under nitrogen protection, add CZ-Br (7.23g, 16.26mmol), 3,6-dihydroxy-9-hydro-carbazole (1.61g, 8.13mmol), cesium carbonate (6.7g, 21.12 mmol), DMF (30 mL). The reaction conditions and post-reaction treatment are the same as the synthesis of CZ-2S1O in Example 1. The product CZ-CZO was obtained with a yield of 60%.
[0059] Step 3, Synthesis of C3
[0060] Add CZ-CZO (3.90g, 4.08mmol) and sodium hydride (0.6g, 24.3mmol) to dry te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com