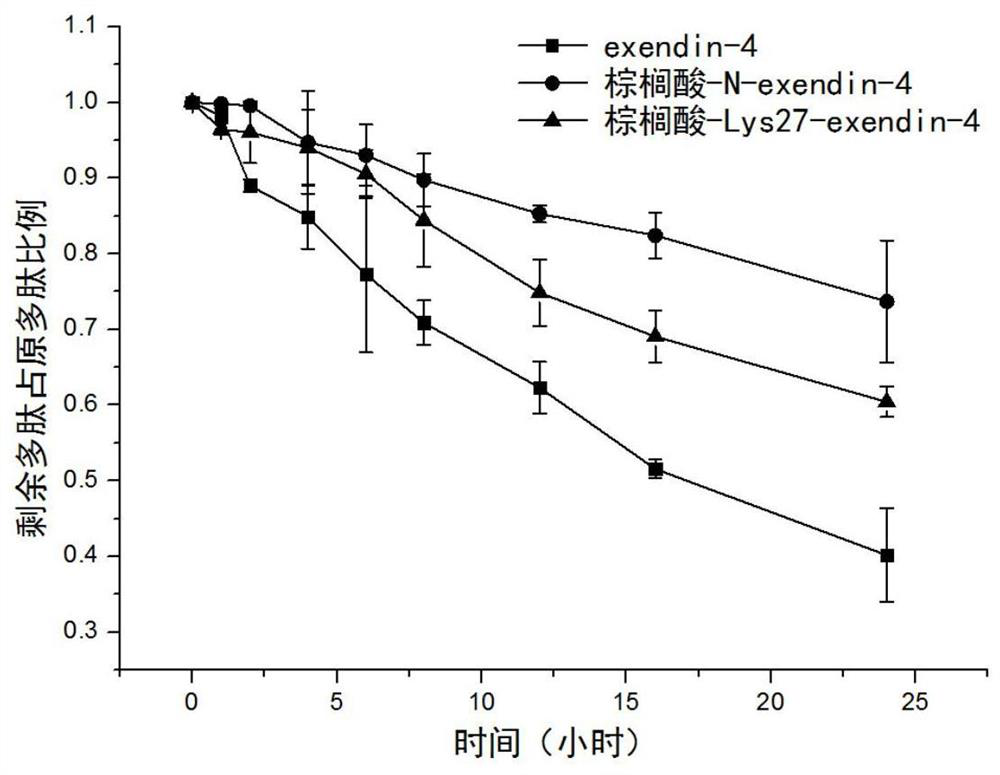
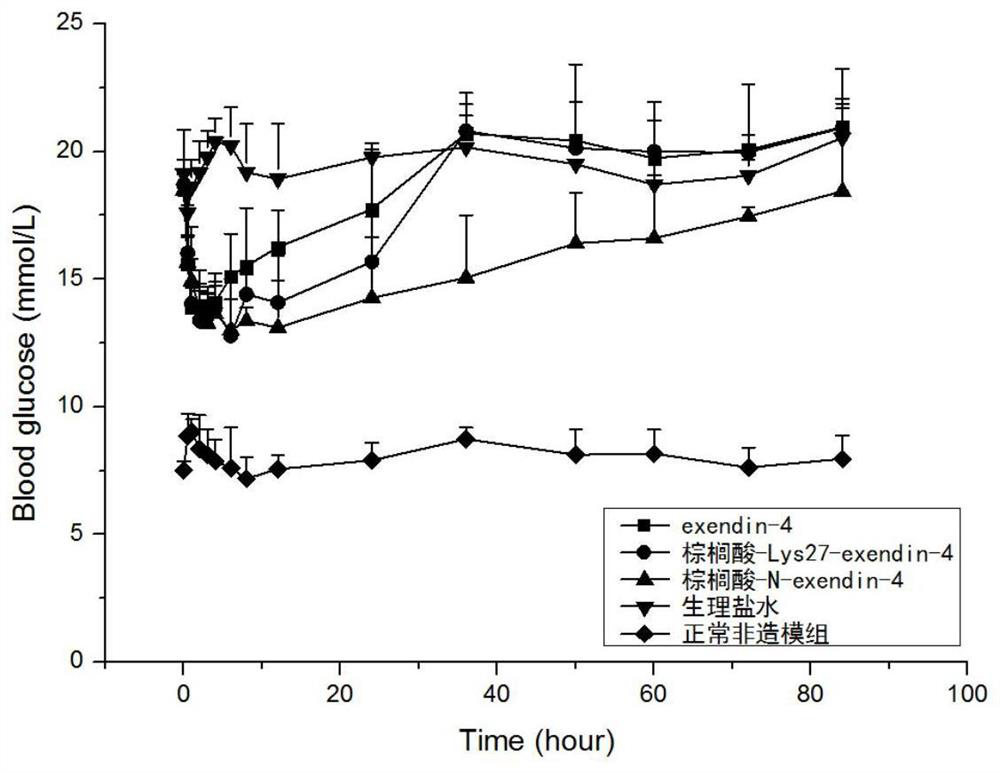
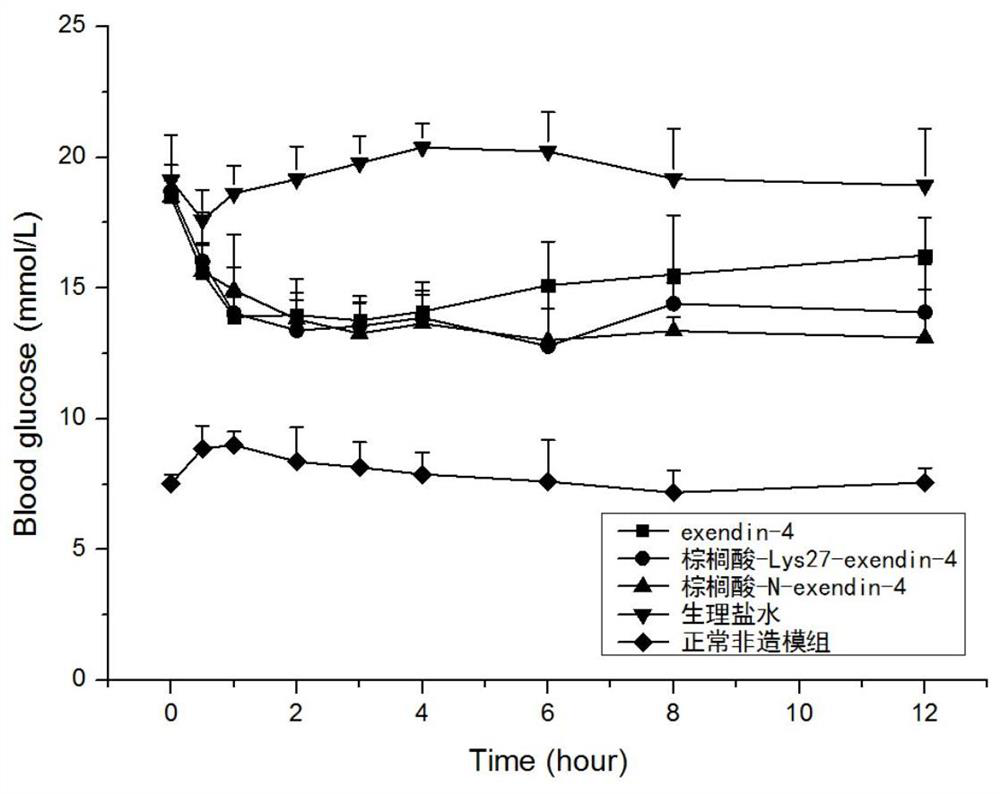
Modifications of exenatide and their applications
A technology of exenatide and modifiers, which is applied in the field of exenatide modifiers, can solve the problems of heavy burden and unsatisfactory effect, and achieve the effects of long half-life, high affinity and activating ability, and long-lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Modifications of Exenatide of the present invention
[0039] The modification of Exenatide provided in this example has the chemical formula:
[0040] (Palmitic Acid) His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile- Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Pro-Ser-NH 2
[0041] Preparation:
[0042] Table 1 The protected amino acids and their abbreviations and English abbreviations required for solid-phase synthesis of exenatide by Fmoc method
[0043]
[0044]
[0045] Exenatide was synthesized by Fmoc solid-phase synthesis, using Fmoc-Rink MBHA Amide resin, using 20% piperidine / N,N-dimethylformamide (DMF) to remove Fmoc, and the coupling reagent was hydroxybenzene Oxytriazole (HOBT) / N,N-diisopropylcarbodiimide (DIC), DMF is the reaction solvent, and the reaction monitoring adopts the ninhydrin detection method. The following protected amino acids are connected to the Rink MBHA Amide ...
Embodiment 2
[0047] Example 2: Modifications of Exenatide of the present invention
[0048] The modification of Exenatide provided in this example has the chemical formula:
[0049] (lauric acid) His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile- Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Pro-Ser-NH 2
Embodiment 3
[0050] Example 3: Modifications of Exenatide of the present invention
[0051] (Palmitic Acid) His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Trp-Glu-Ala-Val-Arg-Leu-Phe-Ile- Glu-Trp-Leu-Lys-Asn-Phe-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Pro-Ser-NH 2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com