Combination of TOLL-like receptor stimulating agent and immune effector cell
A technology of immune effector cells and receptor agonists, applied to receptors/cell surface antigens/cell surface determinants, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0223] Example 1. Preparation of CAR-T cells
[0224] In this example, EGFRvIII is selected as the target of CAR-T cells. In order to more accurately verify the anti-tumor effect in mice, the signal peptide, transmembrane region, and intracellular region selected are of mouse origin. The preparation method is operated according to the conventional CAR-T cell preparation method in the art.
[0225] 1. Retroviral vector construction
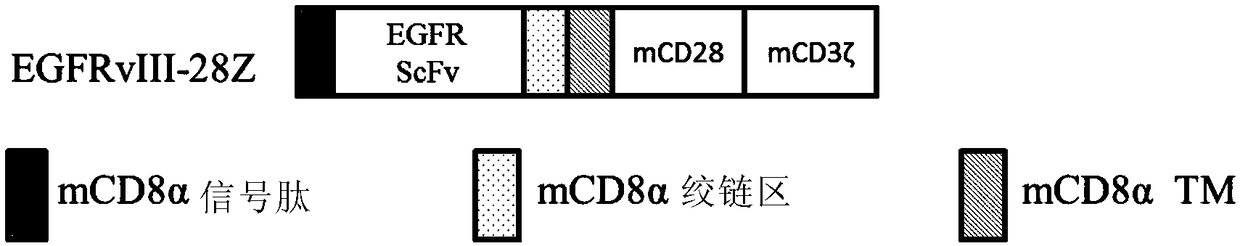
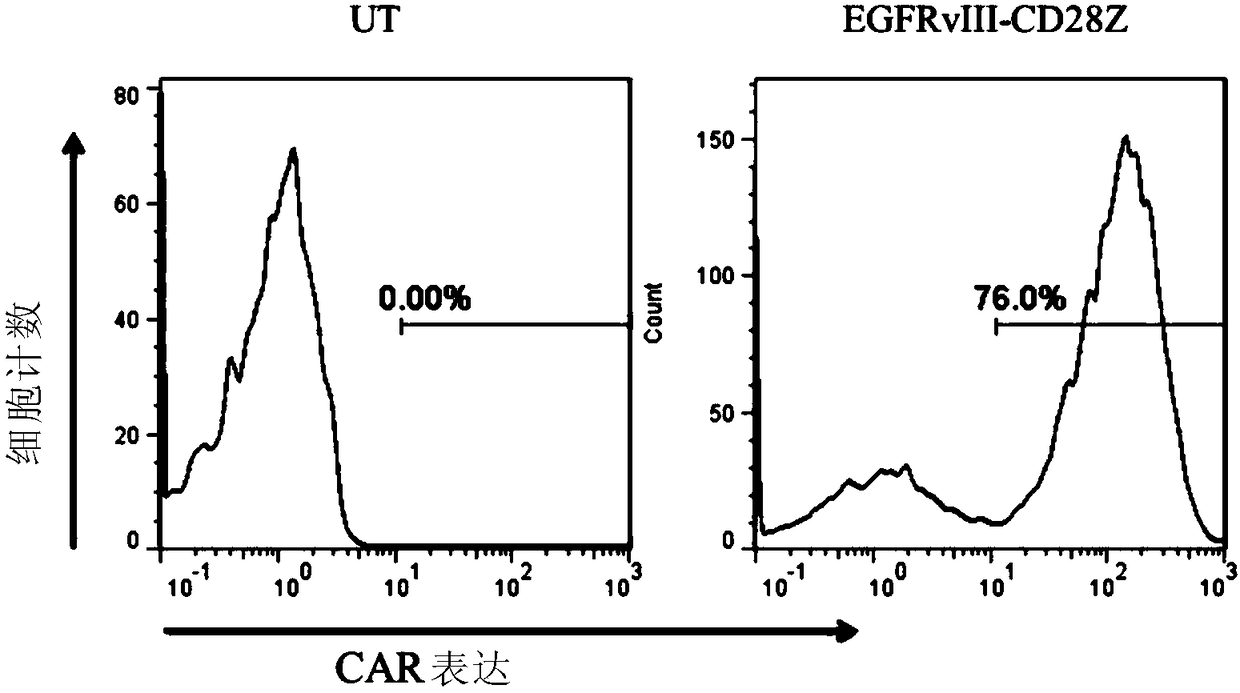
[0226] Mouse CD8α signal peptide (SEQID NO: 1), anti-EGFRvIII monoclonal antibody (SEQID NO: 2), CD8α glue chain region and transmembrane region (SEQID NO: 3), CD28 intracellular domain (SEQID NO: 4) , CD3ζ intracellular domain (SEQID NO: 5) were sequentially connected, and the EGFRvIII-28Z gene fragment was obtained by in vitro gene synthesis, and the IRES- GFP fragment, the recombinant vector MSCV-EGFRvIII-28Z was obtained. The composition sequence of each component fragment is as follows figure 1 shown.
[0227] 2. Preparation of retrovirus...
Embodiment 2
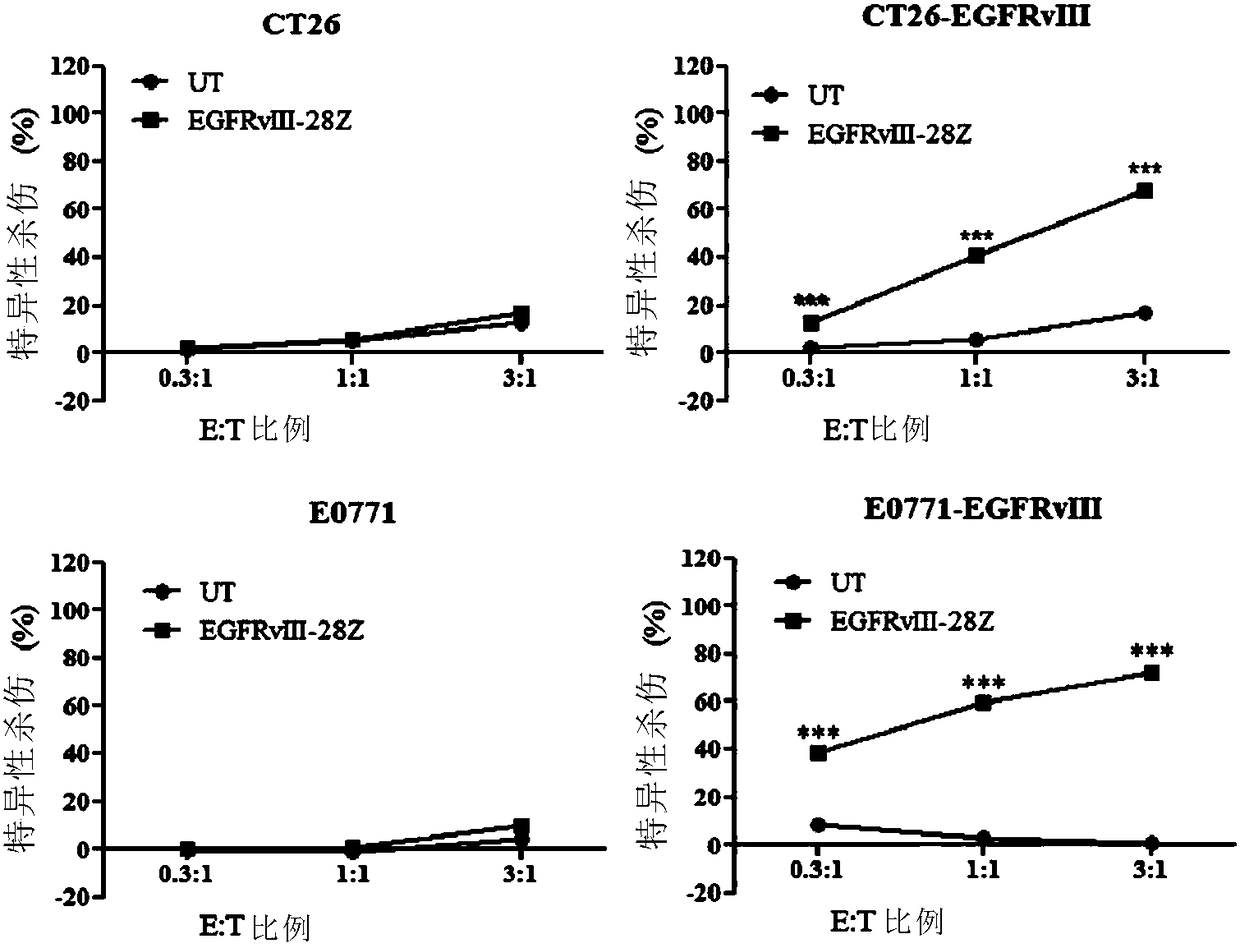
[0238] Example 2. In vitro toxicity of EGFRvIII-28Z CAR T lymphocytes
[0239] Mouse colon cancer cell CT26 (purchased from American Type Culture Collection (ATCC)) and mouse pancreatic cancer cell E0771 (obtained from Baylor College of Medicine) were prepared by conventional techniques in the field to obtain CT26 overexpressing EGFRvIII - EGFRvIII and E0771-EGFRvIII.
[0240] CytoTox 96 non-radioactive cytotoxicity assay kit (Promega, Madison, USA) was used to measure the in vitro cytotoxicity of EGFRvIII-28Z CAR T lymphocytes to target cells. Adjust the concentration of target cells CT26, CT26-EGFRvIII, E0771, and E0771-EGFRvIII to 2×105 / mL, take 50 μL and inoculate them into 96-well plates; inject them into 96-well plates according to the effect-to-target ratio of 0.3:1, 1:1 and 3:1 Add CAR-T cells and control T cells (50ul), set each control well according to the kit instructions, and set 5 duplicate wells for each group; incubate for 18 hours. After incubation, the in v...
Embodiment 3
[0242] Example 3. Anti-tumor effect of EGFRvIII-28Z CAR-T combined with poly I:C in mouse breast cancer model
[0243] Inoculate 1×106E0771-EGFRvIII cells into the fourth pair of mammary gland fat pads of 6-week-old female C57 mice (weight 18g-24g, purchased from Shanghai Lingchang Biotechnology Co., Ltd.); 13 days after tumor inoculation, the tumor-bearing mice Divided into 4 groups, divided into UT cell group, UT+poly I:C group, EGFRvIII-28Z CAR-T cell group and EGFRvIII-28Z CAR-T+poly I:C group, the average tumor volume of each group was about 150mm 3 ; After grouping, infuse 5.0×10 through the tail vein 6 EGFRvIII-28Z CAR-T cells (injection day is day 0), and the U T cell group was used as the control, 50ug polyI:C was injected intratumorally on the 3rd and 6th day after the CAR-T cell reinfusion, and the UT cell group was injected intratumorally same dose of normal saline. Observe the growth of transplanted tumor, the results are as follows: Figure 4A shown.
[0244]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com