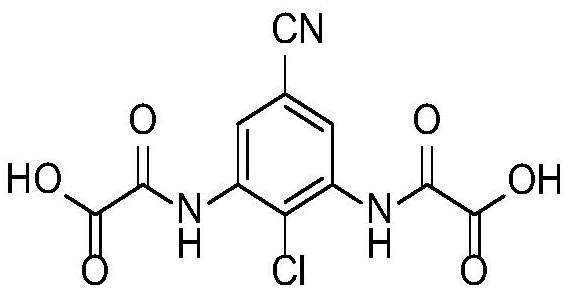
A kind of preparation method of lodoxamide tromethamine intermediate
A technology of lodoxamide tromethamine and lodoxamide, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of incomplete acidification, large residue on ignition, and low purity of products, and reduce industrial waste liquid The production, route safety and environmental protection, the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
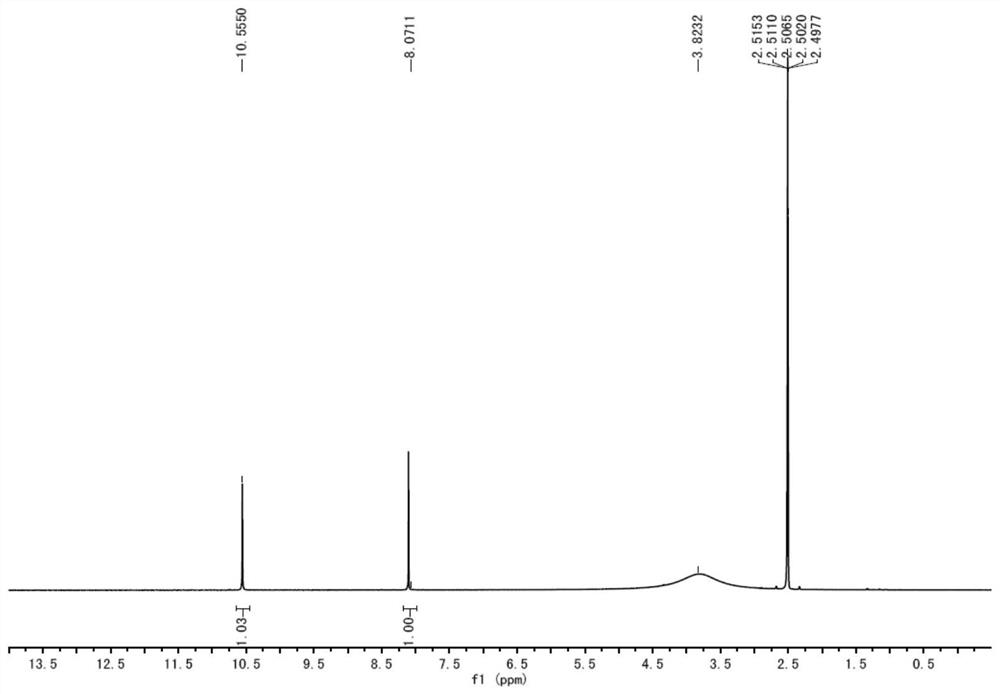
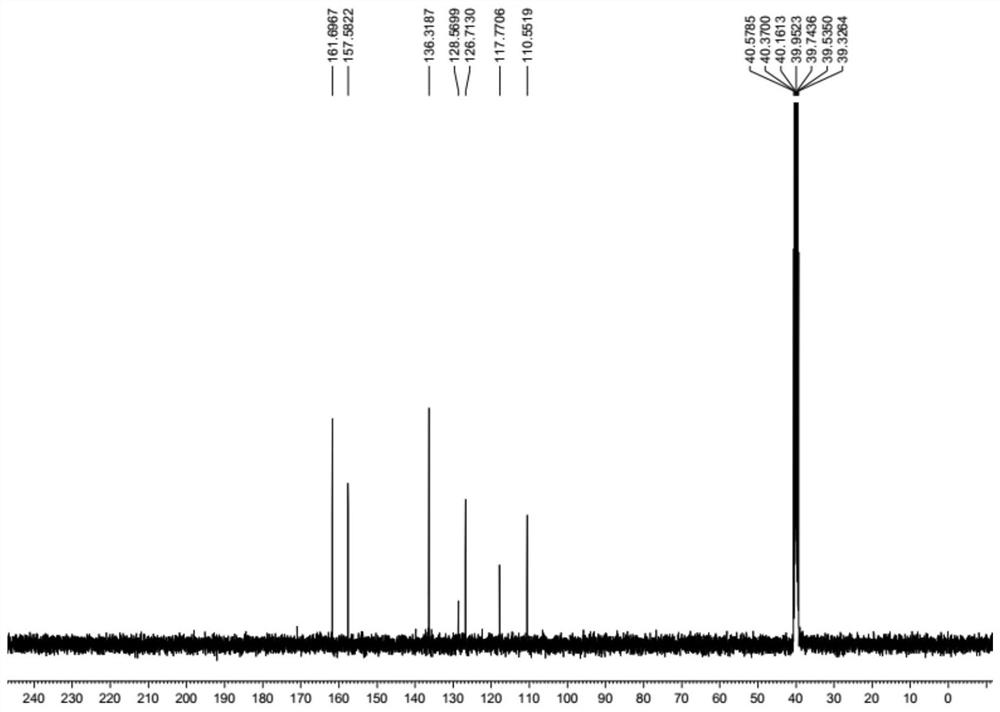
[0034] A preparation method of lodoxamide tromethamine intermediate lodoxamide, the steps are as follows:
[0035] Add 1.5mol oxalyl chloride and 500ml dichloromethane into a dry 5L three-necked flask, cool down to -10°C to 0°C in an ice-salt bath, and take another 0.60mol 3,5-diamino-4-chlorobenzonitrile (Wuhan Development Co., Ltd. Nuo Pharmaceutical Technology Co., Ltd.) was dissolved in 800ml of dichloromethane and added to a dry 1L constant pressure dropping funnel, and the dichloromethane solution of 3,5-diamino-4-chlorobenzonitrile was slowly dropped into the grass under stirring. In the acid chloride dichloromethane solution, the dropping speed is controlled during the dropping process to ensure that the temperature of the reaction solution is between -10°C and 0°C. After the dropwise addition is completed, TLC traces it. After the reaction of the raw materials is completed, slowly add 2L of ice water to the reaction solution. After the dropwise addition was completed,...
Embodiment 2
[0037] A preparation method of lodoxamide tromethamine intermediate lodoxamide, the steps are as follows:
[0038] Add 1.2mol oxalyl chloride and 500ml ethyl acetate into a dry 5L three-necked flask, cool down to -20°C to -10°C in a low-temperature cooling liquid circulation pump, and take another 0.60mol 3,5-diamino-4-chloro Dissolve benzonitrile in 800ml ethyl acetate and add it to a dry 1L constant pressure dropping funnel, slowly drop the ethyl acetate solution of 3,5-diamino-4-chlorobenzonitrile into ethyl oxalyl chloride acetate while stirring In the solution, control the rate of addition during the dropping process to ensure that the temperature of the reaction solution is at -20°C to -10°C. After the dropwise addition is completed, TLC will track it. After the reaction of the raw materials is completed, slowly add 2L of ice water to the reaction solution. After the addition was completed, continue to stir for 0.5 h and then filter with suction, collect the filter cake ...
Embodiment 3
[0040] A preparation method of lodoxamide tromethamine intermediate lodoxamide, the steps are as follows:
[0041] Add 1.8mol oxalyl chloride and 500ml DMF into a dry 5L three-necked flask, cool down to -10°C to 0°C in an ice-salt bath, and dissolve another 0.60mol 3,5-diamino-4-chlorobenzonitrile in 800ml DMF Add it into a dry 1L constant pressure dropping funnel, slowly drop the DMF solution of 3,5-diamino-4-chlorobenzonitrile into the DMF solution of oxalyl chloride under stirring, and control the dropping speed during the dropping process to ensure the reaction The temperature of the solution is -10°C ~ 0°C. After the dropwise addition is completed, TLC traces. After the reaction of the raw materials is completed, slowly add 2L of ice water to the reaction solution. After the dropwise addition, continue to stir for 0.5h and then filter with suction to collect the filter cake. After drying in a blast oven at 60°C, 173.8 g of off-white solid lodoxamide was obtained, with a y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com