Application of arglabin in preparation of drugs for Stargardt disease
A macular degeneration and application technology, applied in sensory diseases, drug combinations, pharmaceutical formulations, etc., to achieve the effects of high safety factor, high safety, and convenient administration methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
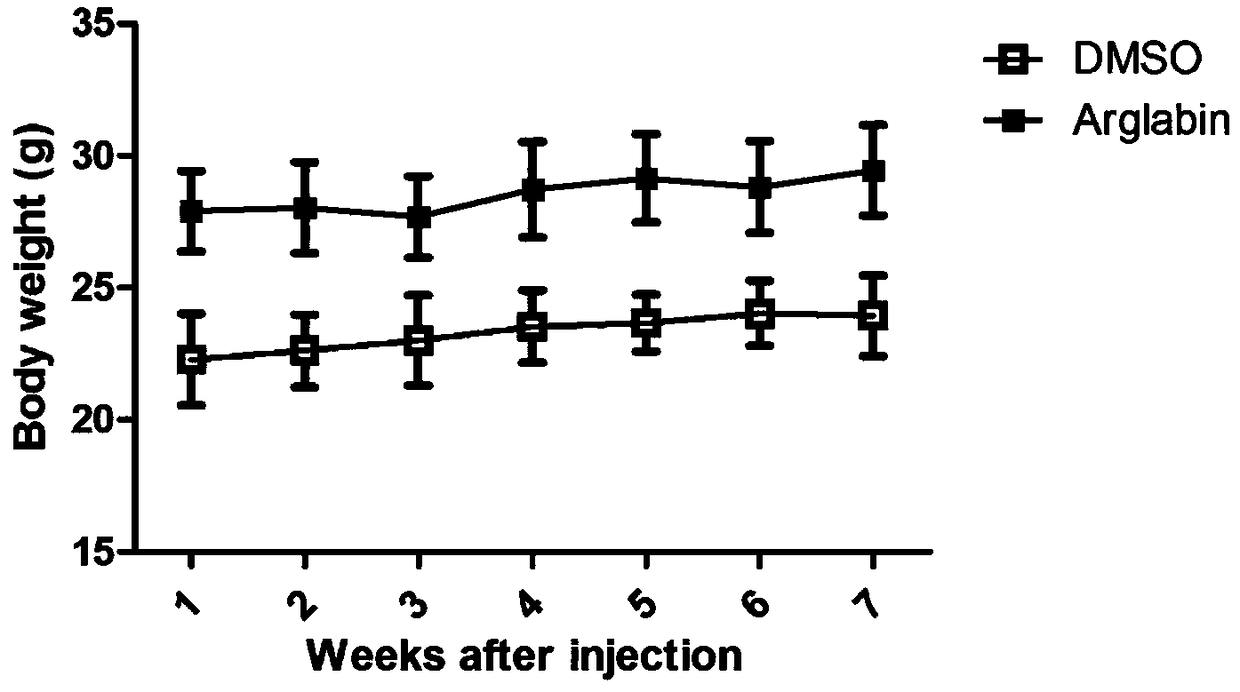
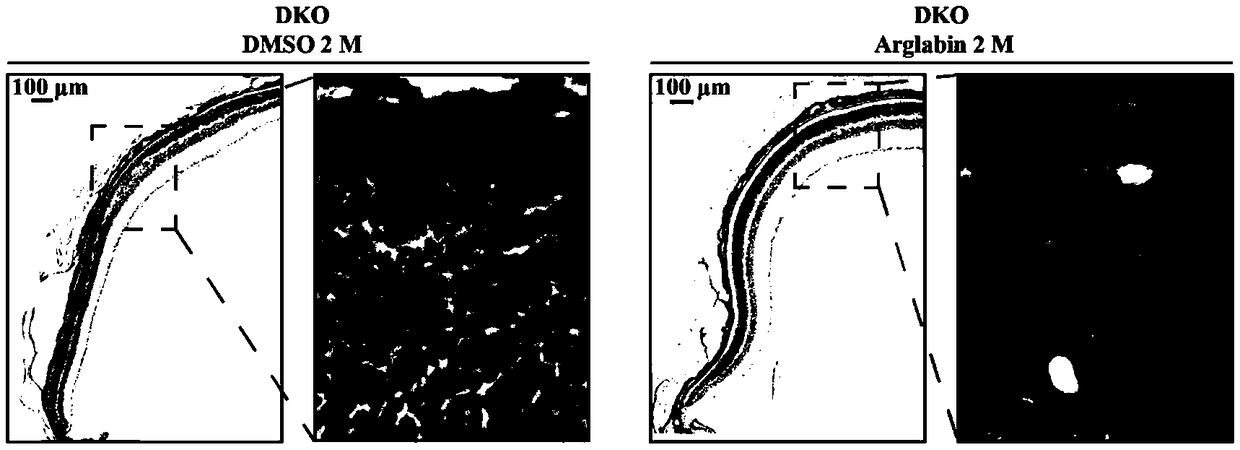
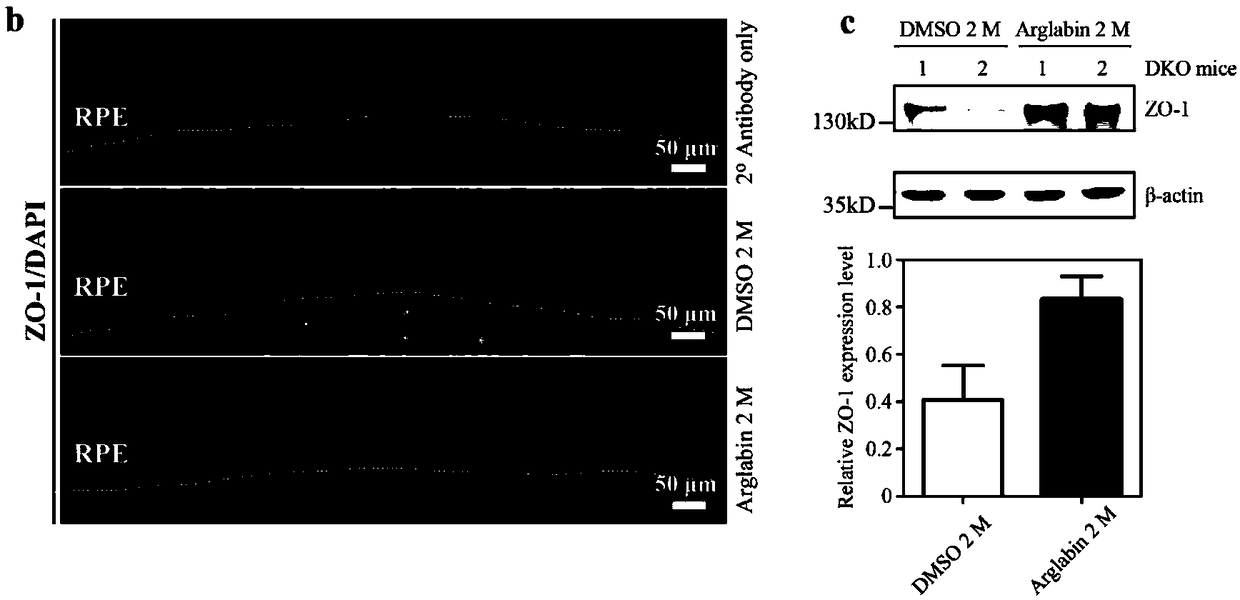
[0029] Abca4 gene knockout mice are mostly used in the development of therapeutic drugs for STGD1. However, unlike STGD1 patients, in single-gene knockout mice, the degeneration of the retina is slow, and the more obvious pathological phenotypes generally appear after 8 months of age, which is not consistent with the phenotype of STGD1 juvenile onset. Therefore, in 2008 the laboratory of Krzysztof Palczewski reported the production of Abca4- / - R8 - / - Double-knockout mice have obvious STGD1 pathological changes such as shortening of the outer segment of photoreceptor cells, thinning of the outer nuclear layer, and accumulation of A2E at the age of 4 months, which can be used as an animal model of STGD1 disease. This embodiment adopts Abca4 - / - R8 - / - Double knockout mice were performed.
[0030] Agalabi was dissolved in DMSO to make 25ng / μL stock solution. Choose 2 months old Abca4 - / - R8 - / - Double-knockout mice, 6 mice were used as the agaleb treatment group, the mother...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com