Adsorbent for hemoperfusion and aqueous solution to remove endotoxin and preparation method thereof
A blood perfusion and aqueous solution technology, applied in chemical instruments and methods, blood circulation treatment, other chemical processes, etc., can solve the problems of low ligand content and low endotoxin adsorption performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
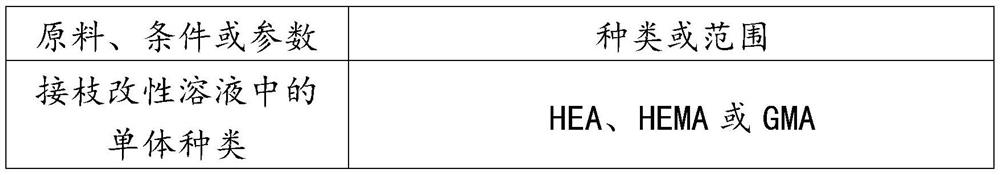
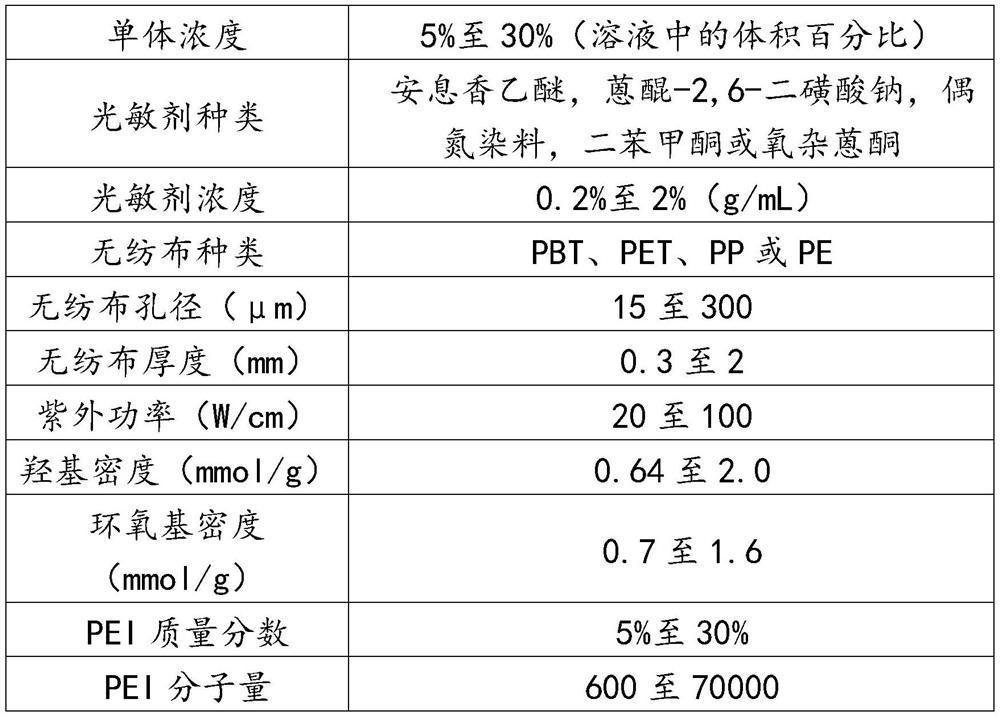
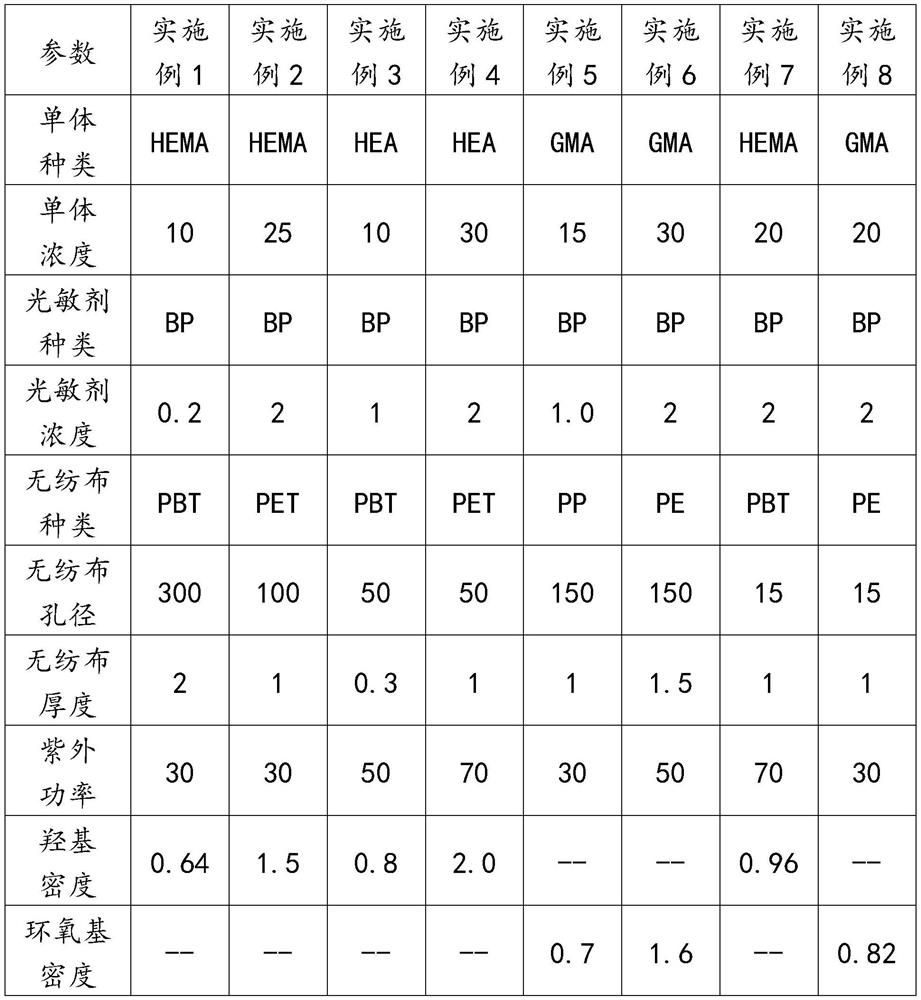
[0039] 1) Grafting solution configured for ultraviolet irradiation grafting: configure the grafting solution of HEMA, BP and BA, wherein the volume fraction of HEMA is 10%, the volume fraction of BA is 90%, and the concentration of BP is 0.2g / 100mL, stir to dissolve evenly, set aside.
[0040] 2) Fully wet the non-woven fabric with the grafting solution: A polybutylene terephthalate (PBT) disc with a pore size of 300 μm, a thickness of 2 mm, and a diameter of 10 cm was immersed in excess grafting solution for 3 minutes.
[0041] 3) UV radiation grafting modified non-woven fabric: take out the non-woven fabric fully wetted by the grafting solution from the grafting solution, drain it at room temperature for 3 minutes, and place it under a UV light source under ventilated conditions. UV radiation grafting reaction for 20 minutes. The main peak of the ultraviolet light source is 365nm, the power is 30W / cm, and the vertical distance from the ultraviolet light source to the non-w...
Embodiment 2
[0044] 1) Grafting solution configured for UV irradiation grafting: configure the grafting solution of HEMA, BP and BA, wherein the volume fraction of HEMA is 25%, the volume fraction of BA is 75%, and the concentration of BP is 2g / 100mL , stir to dissolve evenly, set aside.
[0045] 2) Fully wet the non-woven fabric with the grafting solution: A polyethylene terephthalate (PET) disc with a pore size of 100 μm, a thickness of 1 mm, and a diameter of 10 cm was immersed in excess grafting solution for 3 minutes.
[0046] 3) UV radiation grafting modified non-woven fabric: take out the non-woven fabric fully wetted by the grafting solution from the grafting solution, drain it at room temperature for 3 minutes, and place it under a UV light source under ventilated conditions. UV radiation grafting reaction for 40 minutes. The main peak of the ultraviolet light source is 365nm, the power is 30W / cm, and the vertical distance from the ultraviolet light source to the non-woven fabric...
Embodiment 3
[0049] 1) Grafting solution configured for ultraviolet irradiation grafting: configure the grafting solution of HEA, BP and BA, wherein the volume fraction of HEA is 10%, the volume fraction of BA is 90%, and the concentration of BP is 1.0g / 100mL, stir to dissolve evenly, set aside.
[0050] 2) Fully wet the PBT non-woven fabric with the grafting solution: immerse a PBT disc with a pore size of 50 μm, a thickness of 0.3 mm, and a diameter of 10 cm in the excess grafting solution for 3 minutes.
[0051] 3) UV radiation grafting modified non-woven fabric: take out the non-woven fabric fully wetted by the grafting solution from the grafting solution, drain it at room temperature for 3 minutes, and place it under a UV light source under ventilated conditions. UV radiation grafting reaction for 15 minutes. The main wave peak of the ultraviolet light source is 365nm, the power is 50W / cm, and the vertical distance from the ultraviolet light source to the non-woven fabric disc is 30...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com