Method for preparing astaxanthin by adopting lignocellulose
A lignocellulose and astaxanthin technology, applied in microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve problems such as insufficient efficiency and cost, and difficulty in large-scale application of lignocellulose bioconversion, and achieve The effect of reducing chemical use, lowering the cost of enzymes, and saving water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
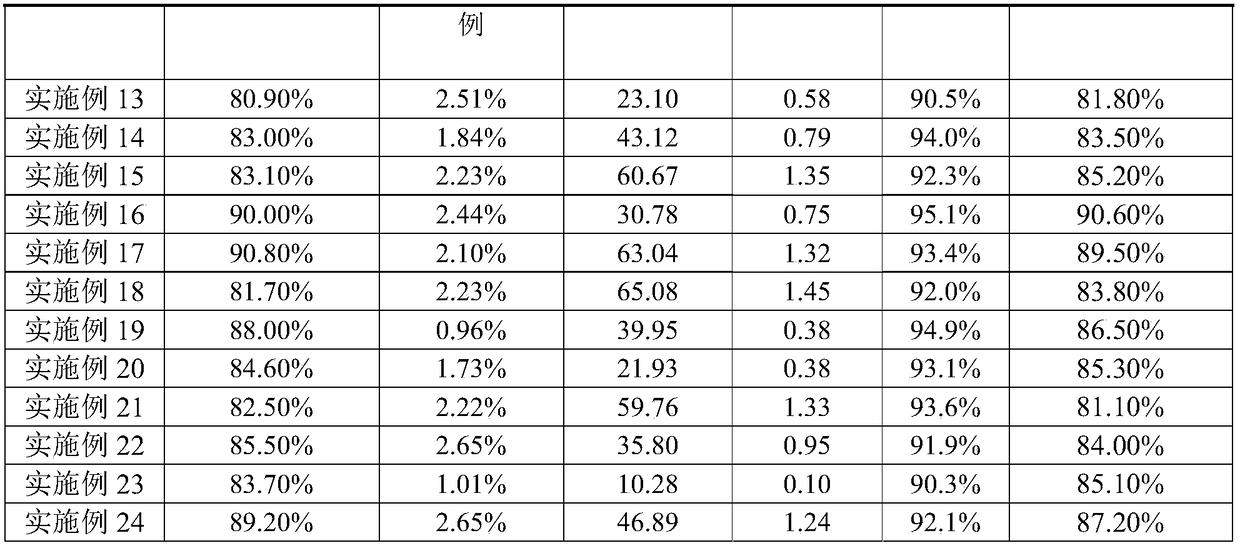
Examples
Embodiment 1
[0049] Embodiment 1: By the method of indirect connection, construct the cellulase preparation based on Clostridium thermocellum cellulite
[0050] Through seamless cloning, the tdk expression cassette (including the promoter of the gapDH gene) and the pyrF expression cassette (including the pyrF self-promoter) were cloned into the plasmid pHK (Mohr, G., Hong, W., Zhang, J., Cui, G.-Z., Yang, Y., Cui, Q., et al. (2013) A targetron system for gene targeting in thermophiles and its application in Clostridium thermocellum, PLoS One 8:e69032.) downstream of the antibiotic gene cat, And through the design of primers, NheI and XbaI restriction sites were added between tdk and pyrF expression cassettes, and EagI and MluI restriction sites were added downstream of pyrF for cloning of homology arm fragments, thereby constructing pHK-HR plasmid.
[0051] The cellulase Cel9K (exocellulase, encoded by the nucleic acid sequence 2113813 to 2111293 in the genome CP002416.1) in the celluloso...
Embodiment 2
[0057] Embodiment 2: By the method of indirect connection, construct the cellulase preparation based on Clostridium thermocellum cellulite
[0058] The difference from Example 1 is that the polypeptide fragment II or polypeptide fragment I was linked to the 3' end of cellulosome endonuclease CelZ (SEQ ID NO: 15). The constructed recombinant strain is cultured to the mid-logarithmic phase in GS-2 medium with 5 grams per liter of cellulose or cellobiose as the carbon source, and can be used as a whole-bacteria enzyme preparation for biosaccharification of lignocellulose.
Embodiment 3
[0059] Embodiment 3: By the method of direct connection, construct the cellulase preparation based on Clostridium thermocellum cellulosome
[0060] Using the overlap extension polymerase chain reaction method, cellulosic exonuclease Cel9-48 (SEQ ID NO: 16) was combined with the sequence of type II adhesion module CohIIct (SEQ ID NO: 7) or type I of Clostridium thermocellum The sequence of the docking module DocIct (SEQ ID NO: 6) was directly connected, wherein the sequence of CohIIct or DocIct was connected to the 3' end of the Cel9-48 sequence to obtain the sequence of Cel9-48-DocIct or Cel9-48-CohIIct.
[0061] Using the Cel9-48-DocIct or Cel9-48-CohIIct sequence as the target sequence, using the MluI and EagI restriction sites to clone into the homologous recombination plasmid pHK-HR, using the lactate dehydrogenase gene clo1313_1160 as the targeted replacement sequence, The homologous recombination plasmid pHK-HR-cel9-48 was constructed. The upstream homology arm HR-up is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com