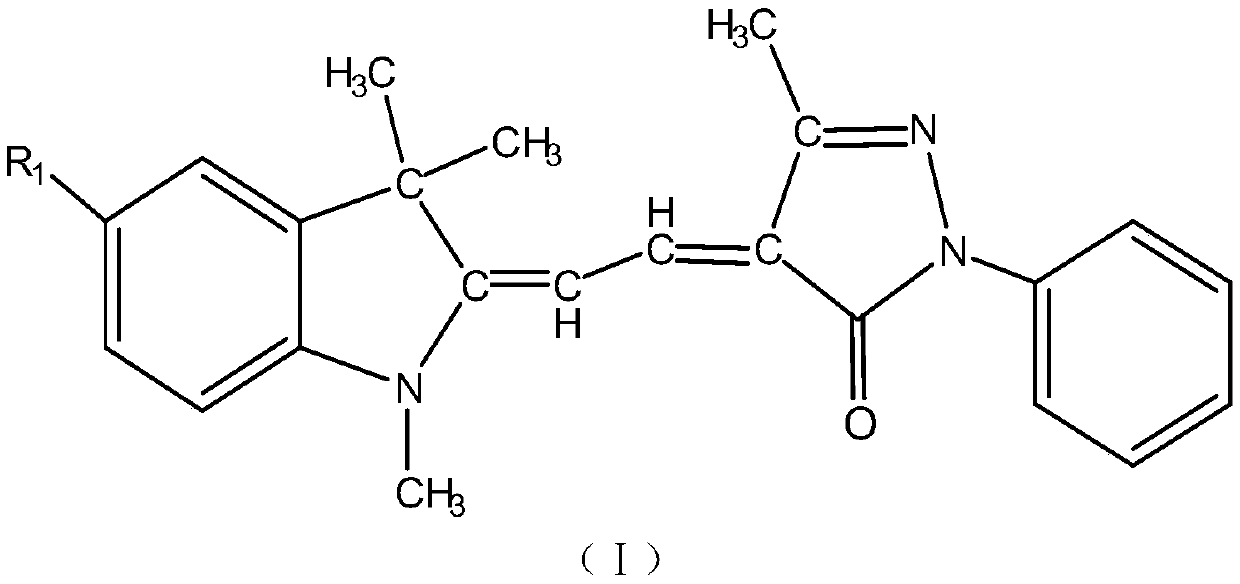
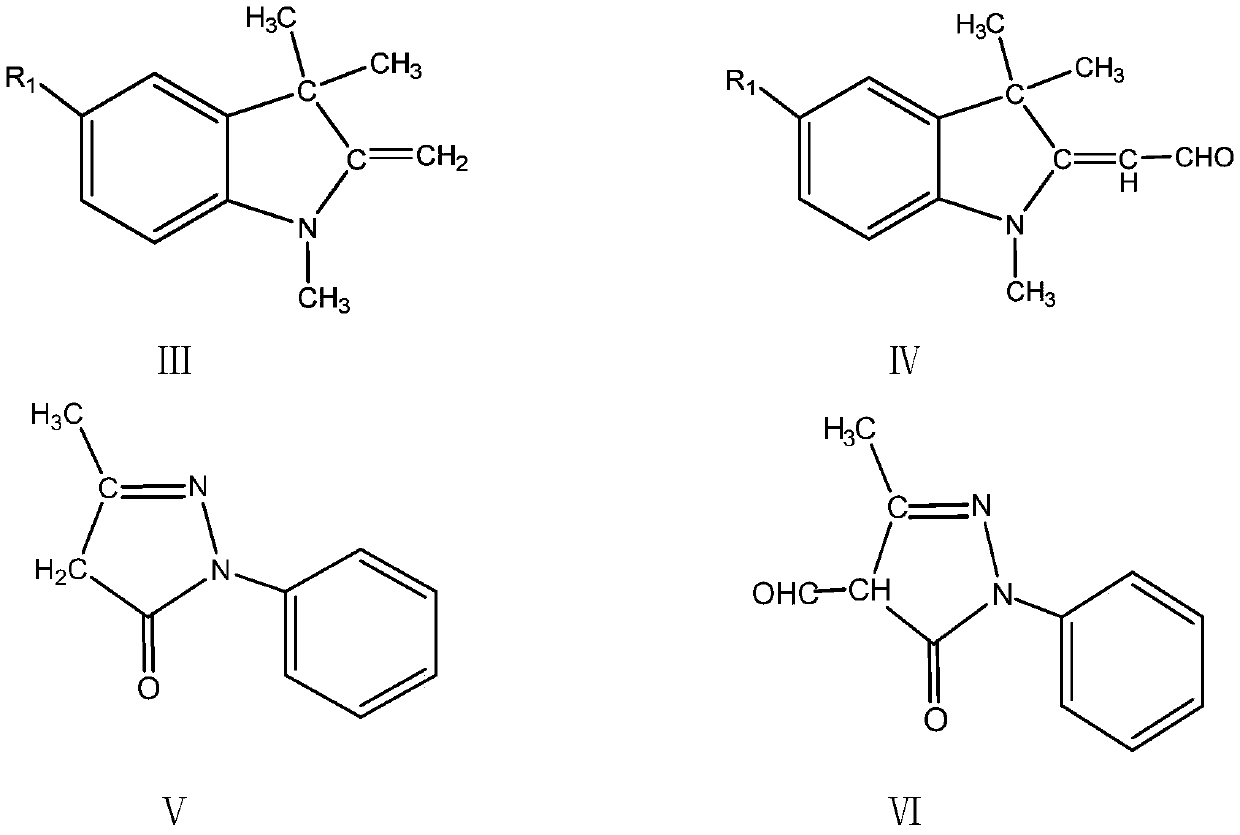
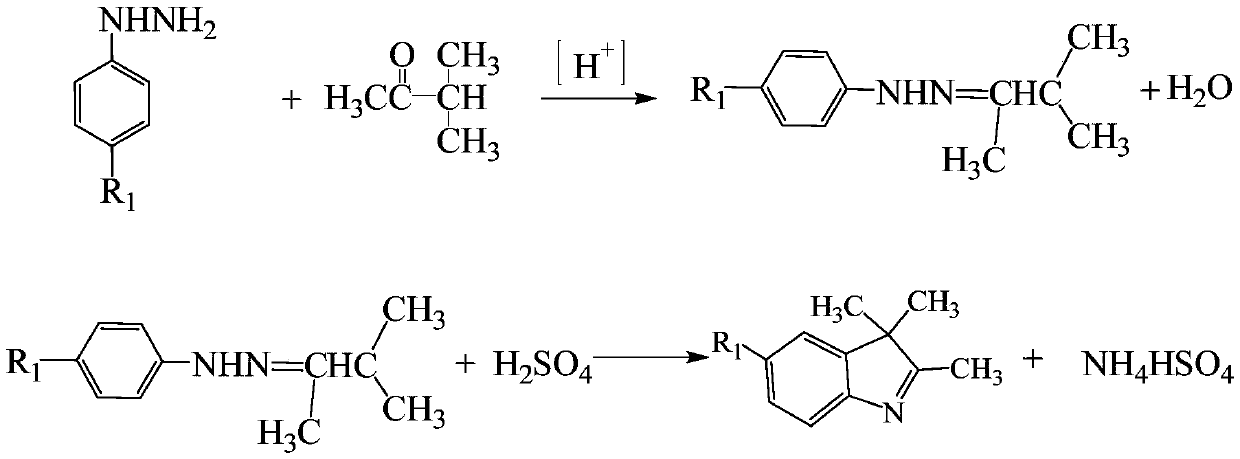
One-pot method for preparing indoline-based methine dyes
A technology of indoline-based dyes, which is applied in the field of preparation of indoline-based methine dyes, can solve the problems of energy consumption and associated distillation residues, difficult procurement of raw materials VI, and rising production costs, achieving smooth undertaking and operability And the effect of stability and high conversion rate of the main reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1 (dye Ⅰ-1)
[0063] In a 250mL flask, add 150mL of methanol, 28.3g of 4-carboxyphenylhydrazine hydrochloride (0.15mol), 18g of sulfuric acid (0.18mol), 13.3g of methyl isopropyl ketone (0.155mol), and raise the temperature to slightly reflux for 10~ 12h to the end of the reaction. Add an appropriate amount of sodium bicarbonate to neutralize, filter, wash the filter cake with an appropriate amount of methanol, and merge the methanol cleaning liquid into the mother filtrate.
[0064] Add 17g of sodium bicarbonate to the above-mentioned condensation filtrate, raise the temperature to slight reflux, slowly add 26.5g of dimethyl sulfate (0.21mol) dropwise, after the drop is complete, keep it under reflux for 1-1.5h to the end of the reaction, filter to obtain the methylated solution .
[0065] Weigh 26.8g of 1-phenyl-3-methyl-4-formyl-5-pyrazolone (0.134mol), add to the above methylation solution, add 5g of p-toluenesulfonic acid, heat up to reflux reaction 5 ...
Embodiment 2
[0067] Embodiment 2 (dye Ⅰ-1)
[0068] In a 250mL flask, add 150mL of methanol, 28.3g of 4-carboxyphenylhydrazine hydrochloride (0.15mol), 18g of sulfuric acid (0.18mol), 13.3g of methyl isopropyl ketone (0.155mol), and raise the temperature to slightly reflux for 10~ 12h to the end of the reaction. After cooling, add an appropriate amount of magnesium oxide to neutralize, filter, wash the filter cake with methanol, and merge the methanol cleaning liquid into the mother filtrate.
[0069] Add 4.4g of magnesium oxide to the above-mentioned condensation filtrate, raise the temperature to slight reflux, slowly add 26.5g of dimethyl sulfate (0.21mol) dropwise, after the dropwise completion, reflux for 1-1.5h to the end of the reaction, and filter to obtain the methylated solution.
[0070] Weigh 27g of 1-phenyl-3-methyl-4-formyl-5-pyrazolone (0.135mol), add it to the above methylation solution, add 5g of p-toluenesulfonic acid, heat up to reflux for 5~ 6h later to the end of the...
Embodiment 3
[0071] Embodiment 3 (dye Ⅰ-1)
[0072] Replace "5g p-toluenesulfonic acid" with "5g acetic acid", and the rest are the same as in Example 1 to obtain 57.9g of the target dye of formula I-1 (yield 86.7%, compared with the standard product, ΔC 0.08, ΔE 0.25, intensity 99.7%.).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com