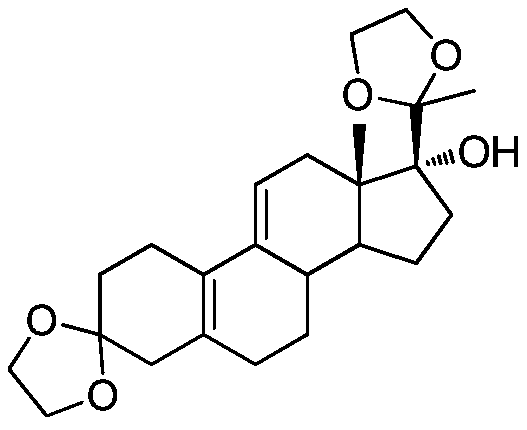
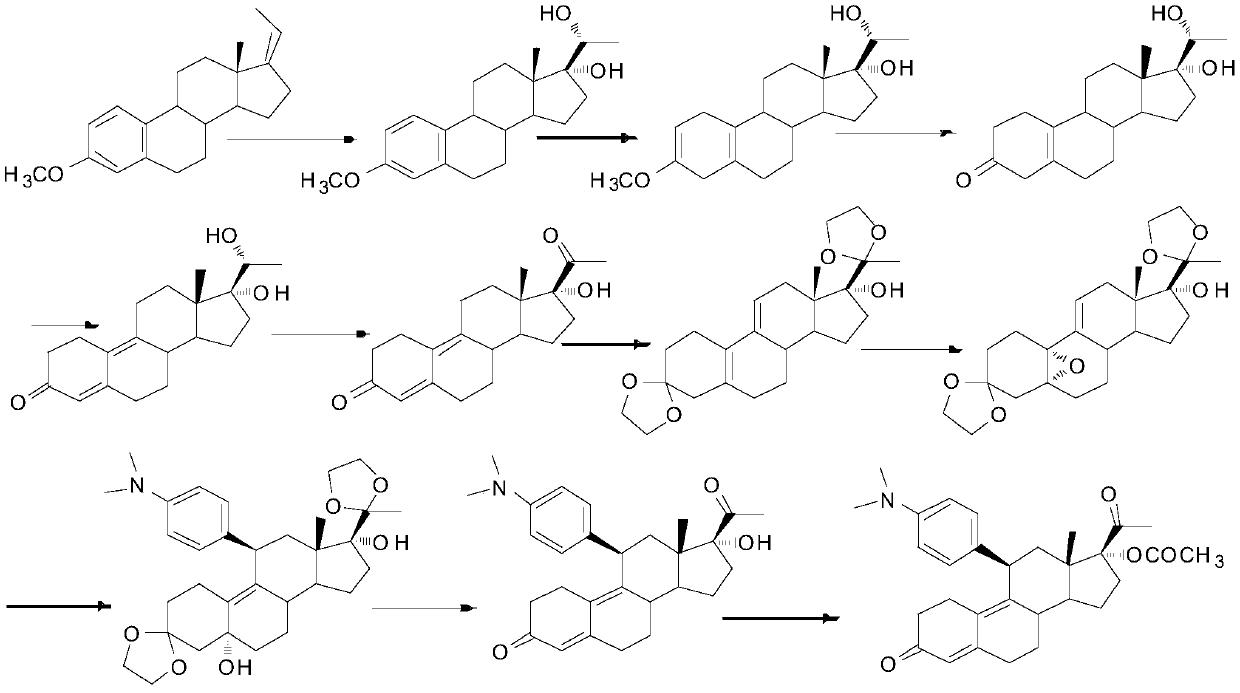
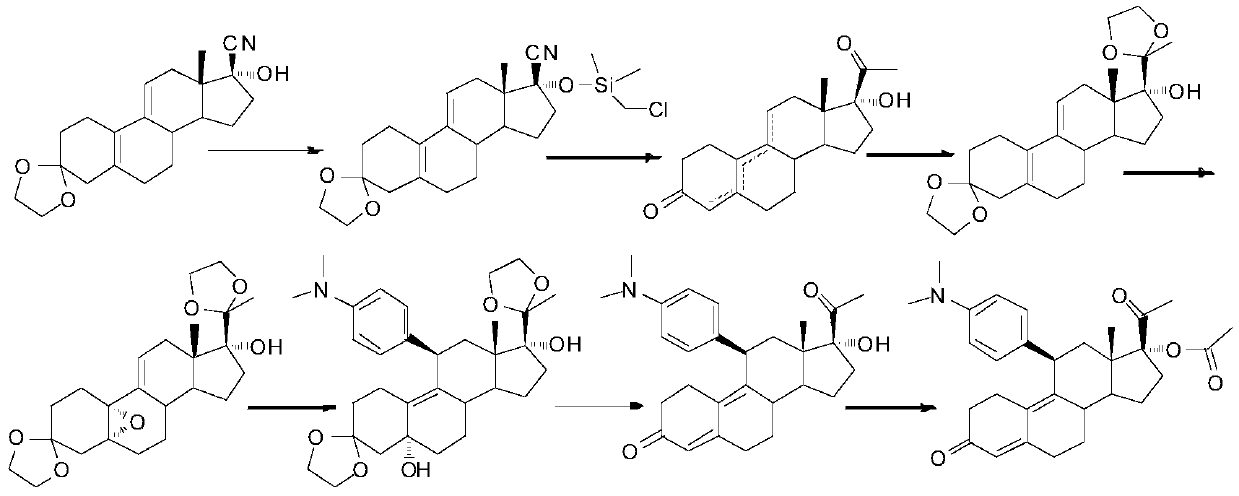
The method for preparing uliplast acetate bisketal
A technology of uliplast acetate and bisketal, which is applied in the direction of ketal steroids, chemical instruments and methods, and steroidal compounds, can solve the problems of single starting raw materials, high cost of raw and auxiliary materials, and large safety hazards, and achieve Increased yield, increased synthesis efficiency, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The first step, ketal reaction: dihydroxyprogesterone dehydrogenate 1 (10g, 1W), ethylene glycol (14ml, 1.4V), triethyl orthoformate (9ml, 0.9V) were dissolved in dichloromethane ( 20ml, 2V), stirred and reacted at 20-25°C, added p-toluenesulfonic acid (0.2g, 0.02W), stirred and reacted at 20-25°C for 2 hours, TLC detected no raw material point, stopped the reaction, and used triethyl The amine was used to adjust the pH to 8, and the solvent was removed by rotary evaporation under reduced pressure, poured into 100ml of ice water for precipitation, stirred, filtered with suction, and dried at 65°C to obtain ketal 2 (11g, 110%), HPLC (240nm, 98 %).
[0060] The second step, aromatic hydrolysis reaction: put benzophenone (15.4g, 1.4W) and ethoxymethane (50.6ml, 4.6V) into the reaction flask to stir, blow nitrogen, cool down to 5-15°C, Add Lithium (5.5g, 0.5W), keep warm at 40-50°C for 0.5 hours, add ketal 2 (11g, 1W) diethoxymethane (22ml, 2V) dropwise, and stir at 80-88°...
Embodiment 2
[0067] The second step, aromatic hydrolysis reaction: put benzophenone (20g, 2W) and tetrahydrofuran (90ml, 9V) into the reaction bottle to stir, blow nitrogen, cool down to 5-15°C, put lithium (12g, 1.2W ), insulated and reacted at 40-50°C for 0.5 hours, added dropwise a solution of ketal 2 (10g, 1W) tetrahydrofuran (20ml, 2V), stirred and reacted at 60-66°C for 2 hours, no raw material point was detected by TLC, and cooled to Stir and add methanol (126ml, 12.6V) dropwise at 15-30°C, then add dropwise 50% dilute hydrochloric acid (concentrated hydrochloric acid (50ml, 5V) + water (50ml, 5V)), heat up to reflux and stir for 2 hours, depressurize Remove the solvent by rotary evaporation, filter with suction, wash the filter cake with hot water above 50°C, wash with ethanol (10ml, 1V), and dry at 60°C to obtain aromatic hydrolyzate 3 (7.5g, 75%), HPLC (240nm, 93%).
[0068] Other steps are with embodiment 1.
Embodiment 3
[0070] The second step, aromatic hydrolysis reaction: put benzophenone (14g, 1.2W) and dimethyltetrahydrofuran (52ml, 5.2V) into the reaction bottle to stir, blow nitrogen, cool down to 5-15°C, and put in lithium (5g, 0.4W), keep warm at 40-50°C for 1 hour, add dropwise ketal 2 (10g, 1W) dimethyltetrahydrofuran (20ml, 2V) solution, stir and react at 80-90°C for 3 hours, TLC detects that there is no raw material point, and the temperature is lowered to 15 to 30°C, and methanol (76ml, 7.6V) is added dropwise with stirring, and then 50% dilute hydrochloric acid (concentrated hydrochloric acid (42ml, 4.2V)+water (42ml, 4.2V)) is added dropwise, Raise the temperature to reflux and stir the reaction for 2 hours, remove the solvent by rotary evaporation under reduced pressure, filter with suction, wash the filter cake with hot water above 50°C, wash with ethanol (10ml, 1V), and dry at 60°C to obtain the aromatic hydrolyzate 3 (7.0 g, 70%), HPLC (240nm, 90%).
[0071] Other steps are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com