Cell reprogramming
A cell and target cell technology, applied in the field of cell reprogramming, can solve the problems of difficult identification of elements, unknown factors, and limited identification of factors that directly reprogram cell type identities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
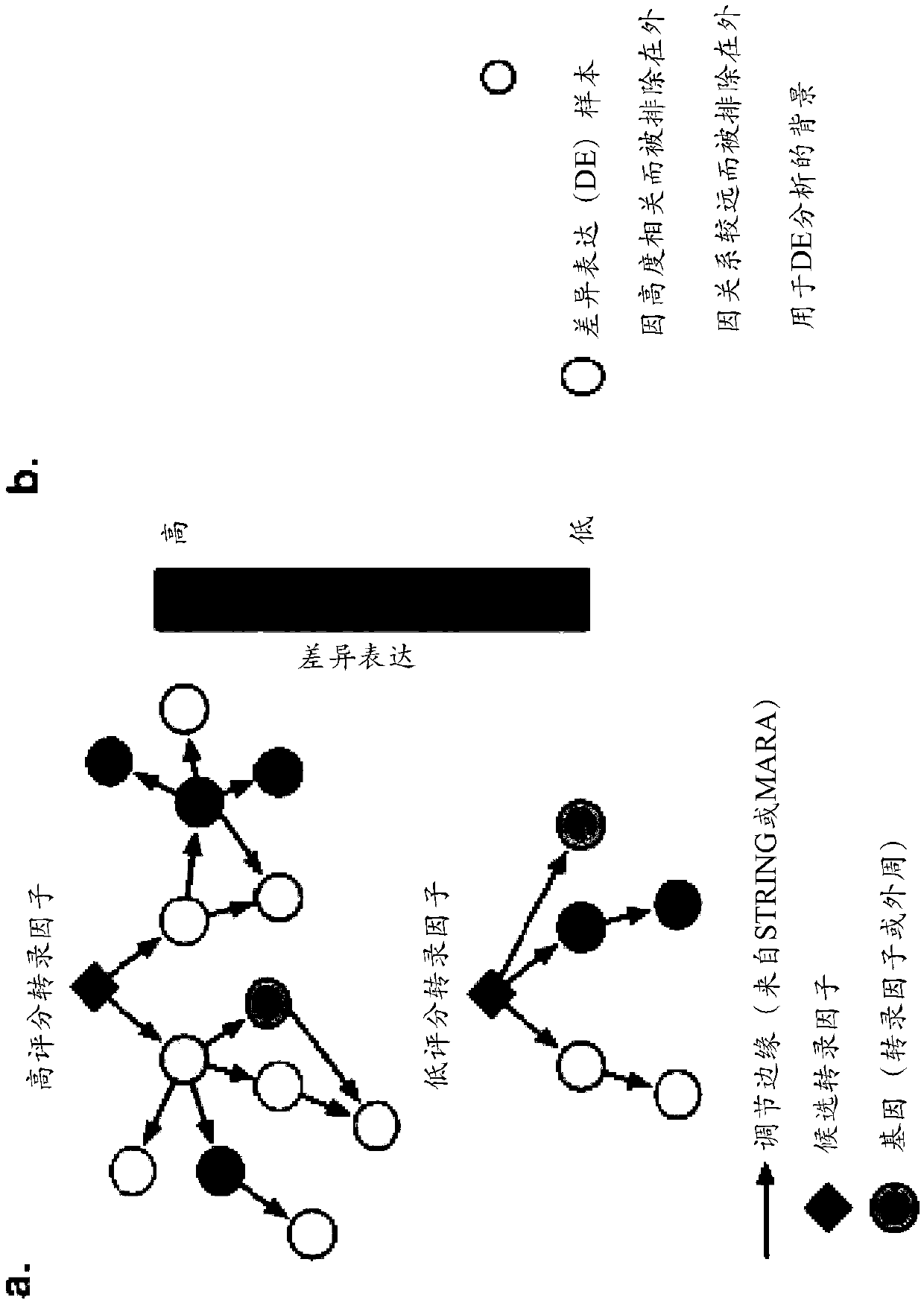
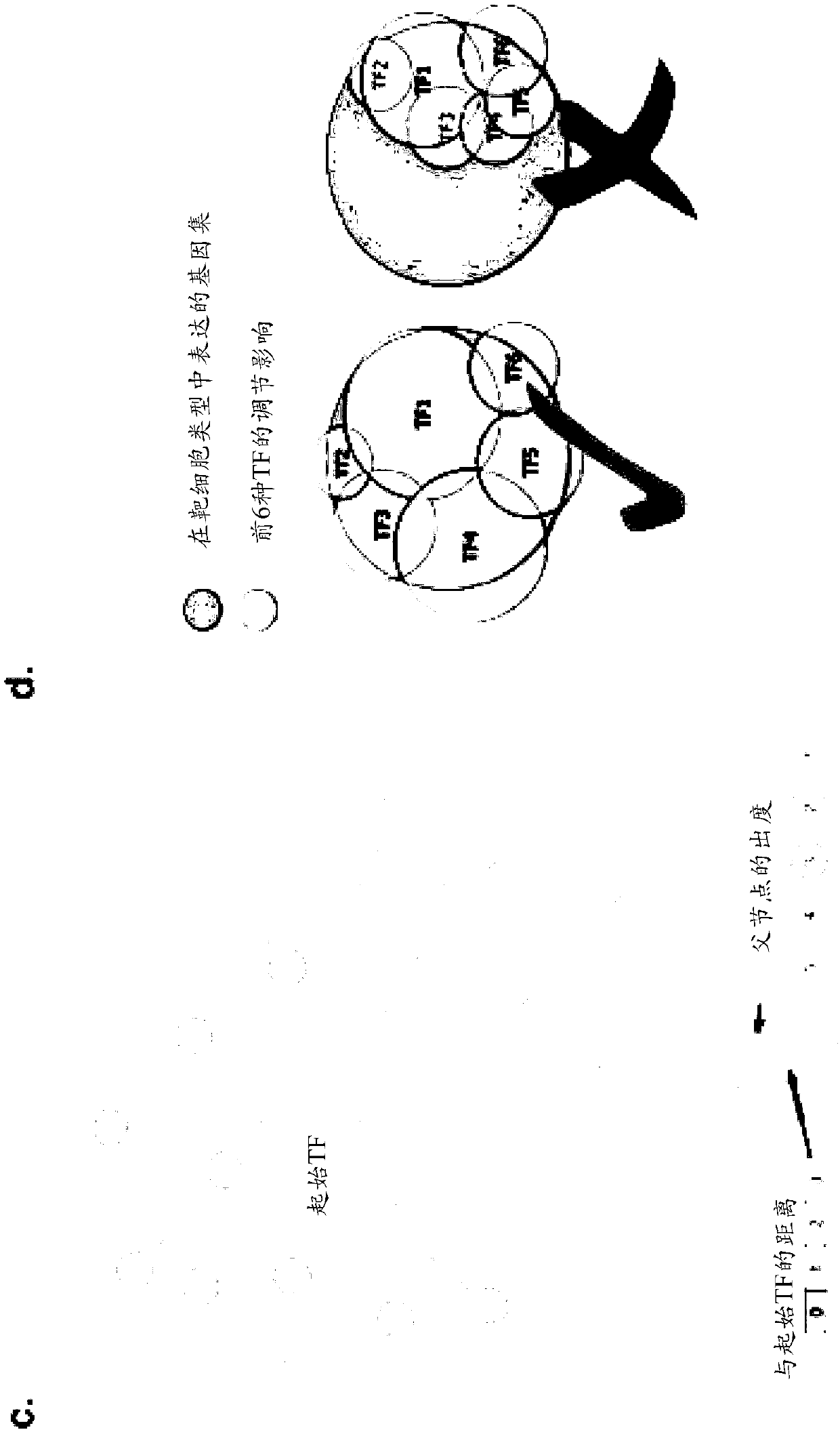
[0406] To predict the set of TFs required for each cell transition, we identified those TFs that were not only differentially expressed between cell types but also exerted regulatory influence on other differentially expressed genes in local networks (see figure 1 a). A single score capturing the differential expression of each gene in each cell type was defined by combining log fold changes and adjusted p-values. By analyzing the known interactome (as defined by STRING and MARA, see figure 1 c) A weighted summation of differential expression scores was performed to calculate the regulatory impact of each TF in each cell type. This sum is weighted by two factors: (1) immediacy by regulation, i.e. how many intermediates are between the TF and downstream genes, and (2) specificity, i.e. the number of other genes that the upstream TF also regulates. This weighted sum allows ranking of TFs in each cell type according to their influence. The final step is to select the best set ...
example 2
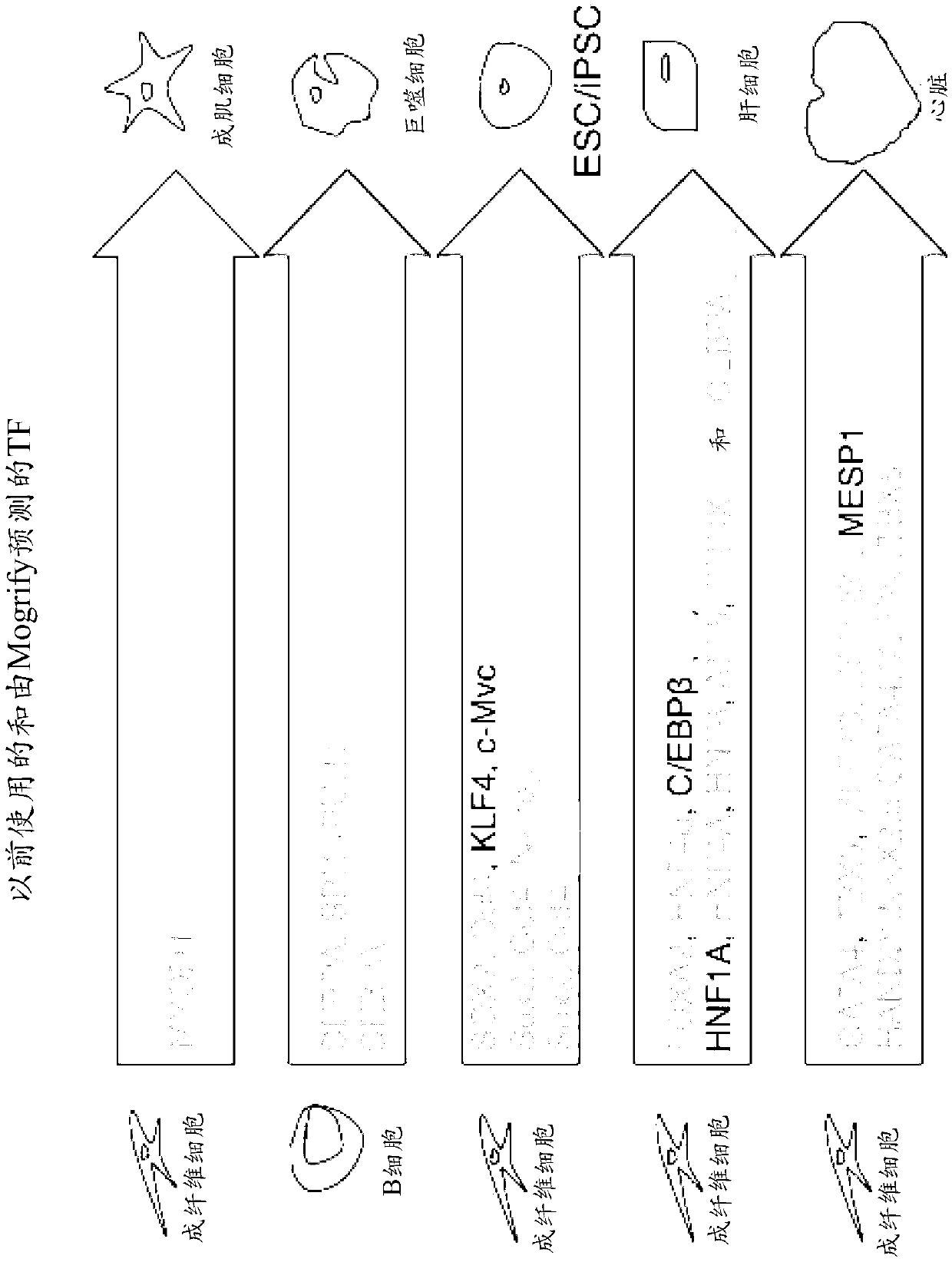
[0445] To assess the predictive power of Mogrify, we first determined how Mogrify counteracts well-known previously published direct cell transformations, focusing on those involving human cells. These should not be considered as absolute perfect combinations, but as positive reference points that can be used for comparison. Such as figure 2 As shown, in almost every case, Mogrify predicts the full set of TFs previously shown to work, but sometimes includes upstream TFs in place of published factors. For example, it is known that human fibroblasts can be converted into iPS cells by introducing OCT4 (also called POU5F1), SOX2, KLF4, and MYC, or OCT4, SOX2, NANOG, and LIN28. Mogrify predicts NANOG, OCT4, and SOX2 as the top 3 TFs for this transition, a combination that has also been experimentally validated. Previous work has demonstrated that expression of CEBPa and PU.1 (also known as SPI1) can switch B cells and fibroblasts into macrophage-like cells (Xie, H., Ye, M., Feng...
example 3
[0466] To empirically demonstrate the predictive power of Mogrify, we performed 11 novel cellular transformations using human cells:
[0467] Fibroblasts to keratinocytes (results in Example 4);
[0468] Keratinocytes to endothelial cells (results in Example 5);
[0469] Fibroblasts to endothelial cells (results in Example 6);
[0470] Embryonic stem cells to endothelial cells (results in Example 7);
[0471] Induced pluripotent stem cells to endothelial cells (results in Example 8);
[0472] Fibroblasts to astrocytes (results in Example 9);
[0473] Embryonic stem cells to astrocytes (results in Example 10);
[0474] Induced pluripotent stem cells to astrocytes (results in Example 11);
[0475] Bone mesenchymal stem cells to astrocytes (results in Example 12);
[0476] Embryonic stem cells to keratinocytes (results in Example 13); and
[0477] • Induction of pluripotent stem cells to keratinocytes (results in Example 14).
[0478] Materials and methods are described i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com