A Fluorescent Probe for Rapid Recognition of Thiophenol
A fluorescent probe and thiophenol technology, which is applied in the field of chemical analysis, can solve the problems of poor water solubility, weak anti-interference ability, and insufficient response speed of fluorescent probes, and achieves strong anti-interference ability, rapid response, and fast response speed. quick effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A method for preparing a fluorescent probe for rapid detection of thiophenol, specifically comprising the following steps:
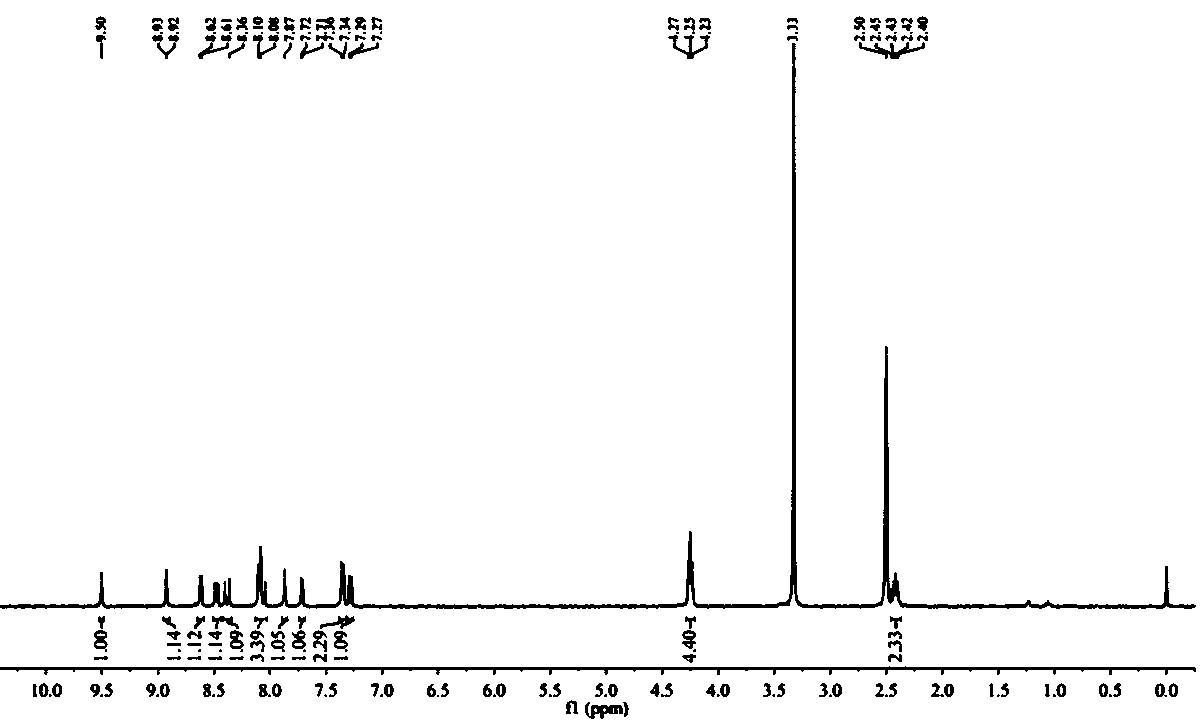
[0038] Synthesis of Intermediate A: Dissolve 1 g (6.84 mmol) of 2,7-naphthyridin-1(2H)-one and 9.81 g (34.21 mmol) of phosphorus oxybromide in 10 mL of acetonitrile and heat to 80 ℃ for 1 h. Add ice water to the reaction system to quench the phosphorus oxybromide, then adjust the pH to neutral. The solution was extracted 3 times with dichloromethane, then with Na 2 SO 4 The dichloromethane extract was dried and distilled under reduced pressure to obtain a red crude product, which was further purified by silica gel column chromatography to obtain a white solid, namely intermediate A (980 mg, yield 69%). 1H NMR (400 MHz, CDC13): δ 9.76 (s, 1H), 8.82 (d, J = 4 Hz, 1H),8.48 (d, J = 4 Hz, 1H), 7.68 (d, J = 8 Hz, 1H), 7.61 (d, J = 8 Hz, 1H).
[0039] Synthesis of Intermediate B: Under argon, 400 mg (1.91 mmol) of Intermediate A and 134 mg (0.19 mmo...
Embodiment 2
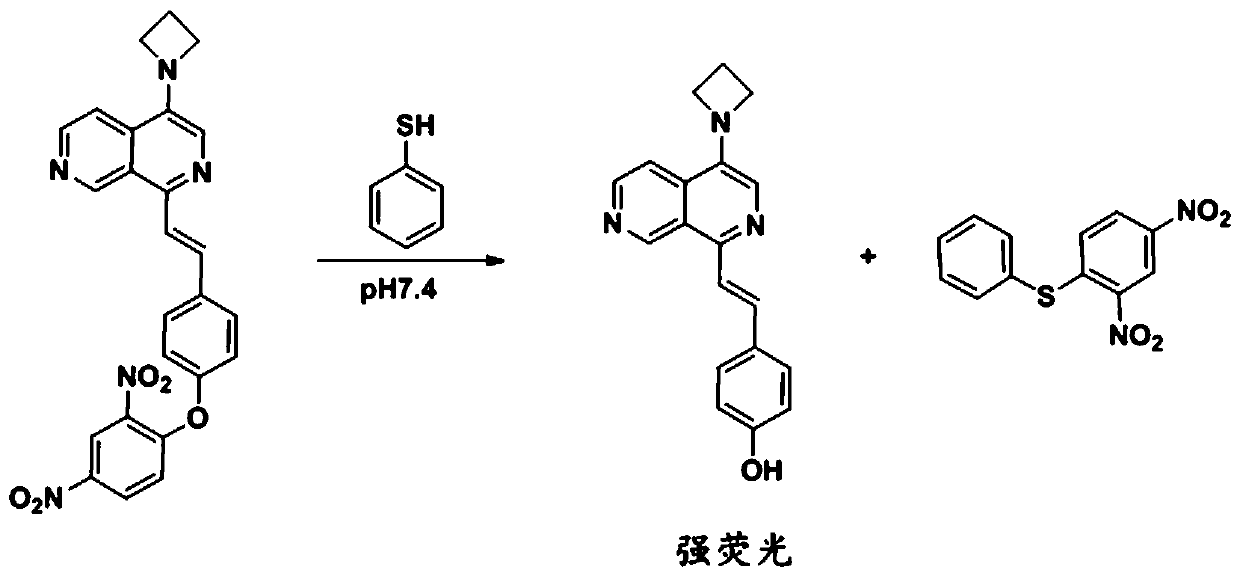
[0046] The effect of the fluorescent probe obtained in Example 1 reacting with thiophenol was determined.
[0047] A 100 μM fluorescent probe solution in Example 1 was prepared using 10 mM PBS (pH=7.4) buffer solution, and a 10 mM thiophenol stock solution was prepared with acetonitrile. Take 100 μL of the above-mentioned fluorescent probe solution and 900 μL PBS (pH=7.4) buffer solution in a cuvette, and detect its fluorescence spectrum under 355 nm excitation, as shown in Figure 4 The solid line part; take 100 μL of the above compound f solution, 895 μL of PBS (pH=7.4) buffer solution and 5 μL of thiophenol stock solution in a cuvette, and detect its fluorescence spectrum under 355 nm excitation, as shown in Figure 4 imaginary part.
[0048] from Figure 4 Comparing the dotted line and the solid line, it can be seen that the background of the fluorescent probe is low and the response to thiophenol is strong, which is a fluorescence-enhanced thiophenol probe.
Embodiment 3
[0050] The analysis of the reaction of the fluorescent probe obtained in Example 1 with different concentrations of thiophenol was determined.
[0051] Add 100 μL of fluorescent probe solution and PBS (pH=7.4) buffer solution to the cuvette, and then add working concentrations of 0, 2, 4, 6, 8, 10, 20, 40, 60, 80, 90 , 100 μM thiophenol was prepared into a 1 mL reaction system, and the fluorescence spectra of different concentrations of thiophenol reacted with the fluorescent probe for 30 seconds were measured, as shown in Figure 5 shown.
[0052] from Figure 5 It can be seen that within a certain concentration range, the fluorescence intensity of the reaction system at 580 nm increases with the concentration of thiophenol, indicating that the probe can detect thiophenol at different concentrations.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com