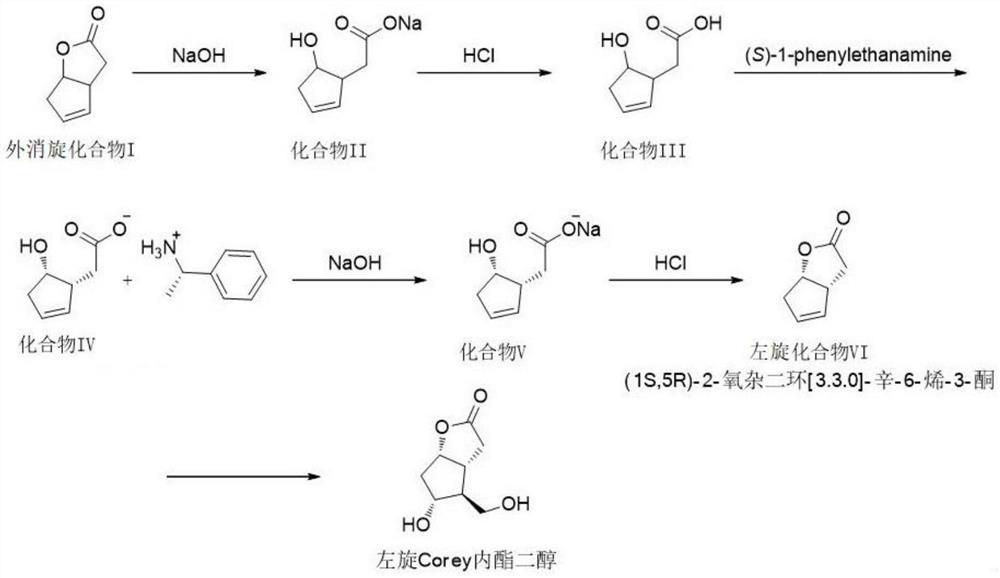
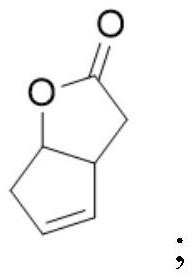
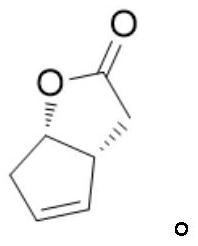
A kind of levorotatory corey lactone diol intermediate, its preparation method and its pharmaceutical application
A technology of lactone diol and intermediate, applied in the field of pharmaceutical preparation, can solve the problems of low enantiomeric yield and low enantiomeric excess, and achieve high enantiomeric yield and high enantiomeric excess. , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Effects of different esterases on the resolution selectivity and activity of compound I.
[0039] In parallel, take ten parts of the prepared buffer solution of disodium hydrogen phosphate and sodium dihydrogen phosphate (pH=8.0) 10mL into a 50mL reaction bottle, and place them in a shaker at 30°C; then add different Enzyme 1g; then weigh 20g of racemic compound I and 69g of vinyl acetate into 100mL of ethyl acetate solution, then divide the above mixed solution into ten parts in parallel and add to the reaction system, start the shaker, and start the reaction. The reaction was sampled at intervals of 1 h. When sampling, it was allowed to stand for stratification, and 500 μL of the upper organic layer was taken out for chiral detection. The results are shown in Table 1.
[0040] Table I
[0041]
[0042] It can be seen from the comparison of the impact of different enzymes on the resolution selectivity and activity in Table 1: the reaction temperature is ...
Embodiment 2
[0043] Example 2: Effects of different reaction temperatures on the resolution selectivity and activity of Lipase AK.
[0044]In parallel, take five prepared buffer solutions of disodium hydrogen phosphate and sodium dihydrogen phosphate (pH=8.0) from 10mL to 50mL in reaction bottles, and place them at 20°C, 30°C, 40°C, 50°C , in a shaker at 60°C; then add 1 g of Lipase AK enzyme to the above system; then weigh 4 g of racemic compound I and 35 g of vinyl acetate to prepare 50 mL of ethyl acetate solution, then divide it into 5 parts in parallel and add to In the reaction system, start the shaker to start the reaction. The reaction was sampled at intervals of 1 h. When sampling, it was allowed to stand for stratification, and 500 μL of the upper organic layer was taken out for chiral detection. The results are shown in Table 2.
[0045] Table II
[0046]
[0047] From Table 2, the comparison of the effects of different reaction temperatures on the resolution selectivity an...
Embodiment 3
[0048] Example 3: Effects of different pH buffer solutions on the resolution selectivity and activity of Lipase AK.
[0049] Prepare buffer solutions of disodium hydrogen phosphate and sodium dihydrogen phosphate with different pH values (pH = 6.0, 7.0, 8.0, 9.0, 10.0), and then measure 10mL to 50mL reaction flasks, and place them at 30°C Then add 1 g of LipaseAK enzyme to the above system; then weigh 4 g of racemic compound I and 35 g of vinyl acetate to prepare 50 mL of ethyl acetate solution, then divide it into 5 parts in parallel and add to the reaction system, Start the shaker and start the reaction. The reaction was sampled at intervals of 1 h. When sampling, the samples were allowed to stand for stratification, and 500 μL of the upper organic layer was taken out for chiral detection. The results are shown in Table 3.
[0050] Table three
[0051]
[0052] It can be seen from the comparison of the effects of different pH buffer solutions in Table 3 on the separat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com