A kind of free radical polymerizable rosin benzocyclobutene monomer, its preparation method and application
A technology of benzocyclobutene and polymerized rosin, applied in the field of rosin benzocyclobutene monomer, can solve problems such as unsatisfactory performance, and achieve the effects of improving use value, reducing use, and strengthening reprocessing and utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The monomer prepared in this embodiment is: 12-position benzocyclobutene dehydroabietic acid allyl alcohol ester, the structure is as follows:
[0047]
[0048] The preparation process is as follows:
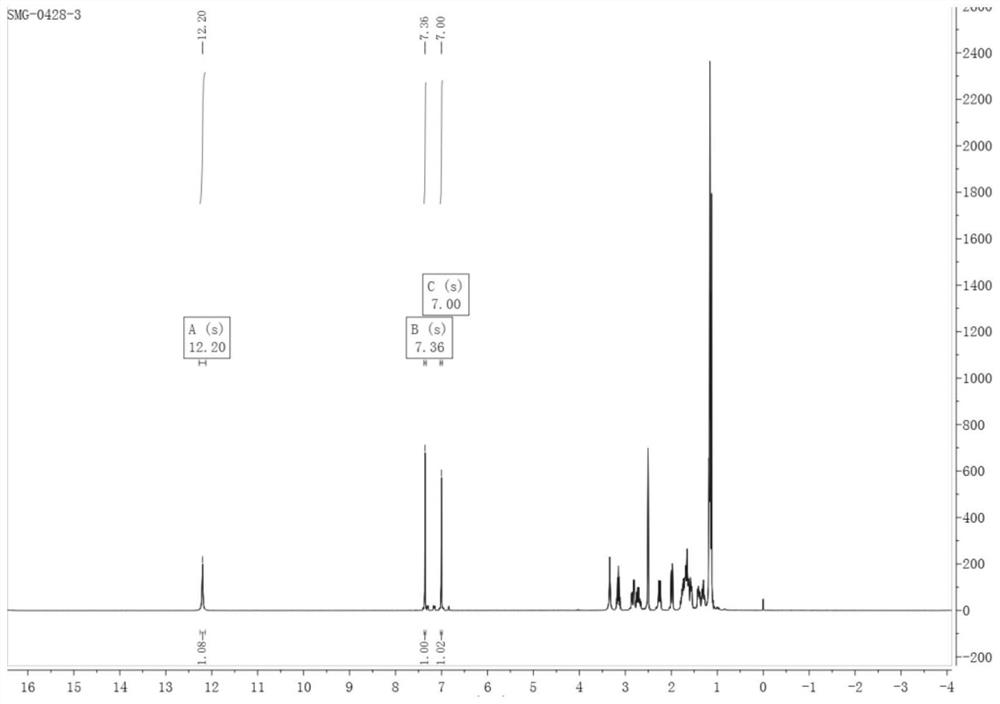
[0049] 1) Add 5.00 g of starting materials dehydroabietic acid, 5.54 g of NBS, and 337 mL of anhydrous acetonitrile into a round-bottomed flask, react at 25°C in the dark for 24 hours, filter with suction, dissolve the solid in ethyl acetate, add H 2 O was extracted, and the aqueous phase was washed with ethyl acetate (50mL×2), and the combined organic phases were washed with H 2 The organic phase was washed with O (50 mL×2); after that, it was washed with anhydrous Na 2 SO 4 Dry the organic phase, filter, and rotary evaporate to obtain a white solid substance: 12-bromodehydroabietic acid, the purity is greater than 95%, and the yield is greater than 90%. The product structure of this step is characterized by: 1H NMR (400MHz, DMSO) δ12.20(s, 1H), 7.36(s, 1H), 7.00(s...
Embodiment 2
[0054] The monomer prepared in this embodiment is: 12-position benzocyclobutene dehydroabietic acid allyl alcohol ester, the structure is as follows:
[0055]
[0056] The preparation process is as follows:
[0057] 1) Add 5.00 g of starting materials dehydroabietic acid, 5.54 g of NBS, and 300 mL of anhydrous acetonitrile into a round-bottomed flask, react at 25°C in the dark for 24 hours, filter with suction, dissolve the solid in ethyl acetate, add H 2 O was extracted, and the aqueous phase was washed with ethyl acetate (50mL×2), and the combined organic phases were washed with H 2 The organic phase was washed with O (50 mL×2); after that, it was washed with anhydrous Na 2 SO 4 Dry the organic phase, filter, and rotary evaporate to obtain a white solid substance: 12-bromodehydroabietic acid, the purity is greater than 95%, and the yield is greater than 90%. The product structure of this step is characterized by: 1 H NMR (400MHz, DMSO) δ12.20(s, 1H), 7.36(s, 1H), 7.00(...
Embodiment 3
[0061] Preparation of monomer
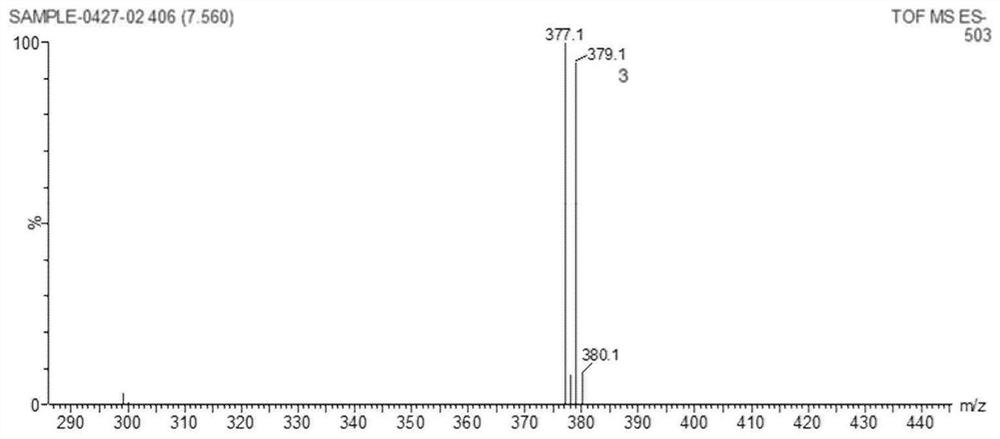
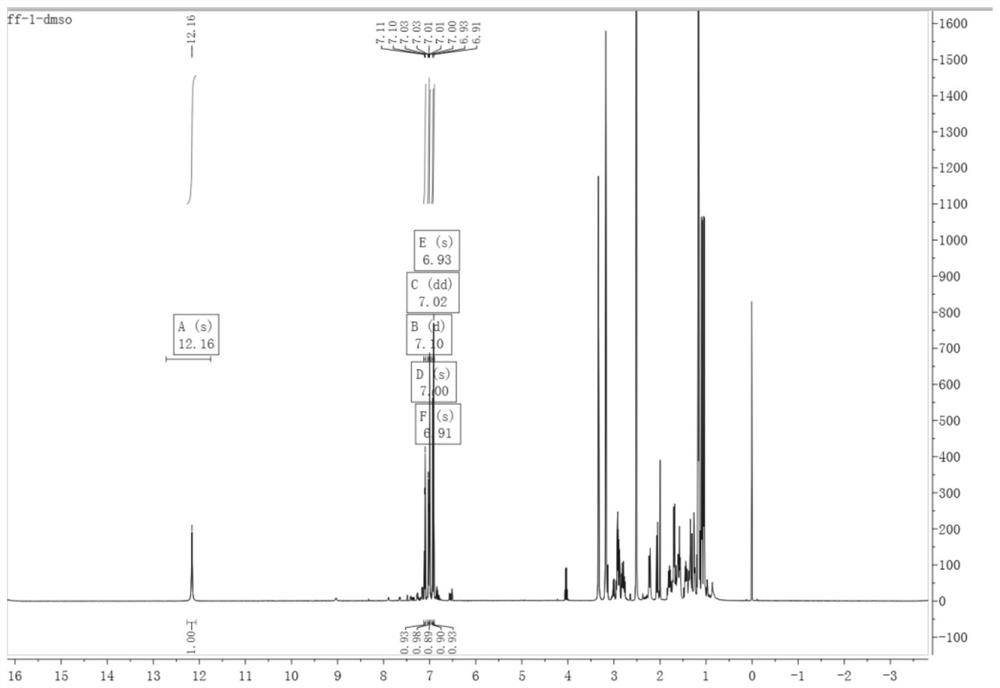
[0062] 12-bromodehydroabietic acid (the product obtained in step 1 of Example 1)) 0.419g, 0.185g of 4-boronic acid benzocyclobutene were dissolved in 10mL of dioxane, and were added to a three-necked flask and then Add potassium carbonate 0.255g, in N 2 Under protection, add tetrakis triphenylphosphine palladium 0.005g; 2 At 75°C, react for 8 hours, cool to room temperature, wash with diatomaceous earth and ethyl acetate, add H 2 O extraction; the aqueous phase was washed (50mL×2) with ethyl acetate, and the combined organic phases were washed with H 2 O washes the organic phase (50 mL×2); after that anhydrous Na 2 SO 4 The organic phase was dried, filtered, and rotary evaporated to obtain a white solid: 12-position benzocyclobutene dehydroabietic acid. The product structure of this step was characterized by: 1 H NMR(500MHz,DMSO)δ12.16(s,1H),7.10(d,J=7.5Hz,1H),7.02(dd,J=7.5,0.9Hz,1H),7.00(s,1H),6.93 (s,1H),6.91(s,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com