A dehydroabietyl-3,4-dihydropyrimidin-2(1h)-one derivative, its preparation method and application
A technology of dihydropyrimidine and derivatives, applied in organic chemistry, antiviral agents, etc., can solve the problems of low yield, many side reactions, harsh reaction conditions, etc., achieve high yield, strengthen reprocessing and utilization, and post-treatment easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of compound a:
[0048]
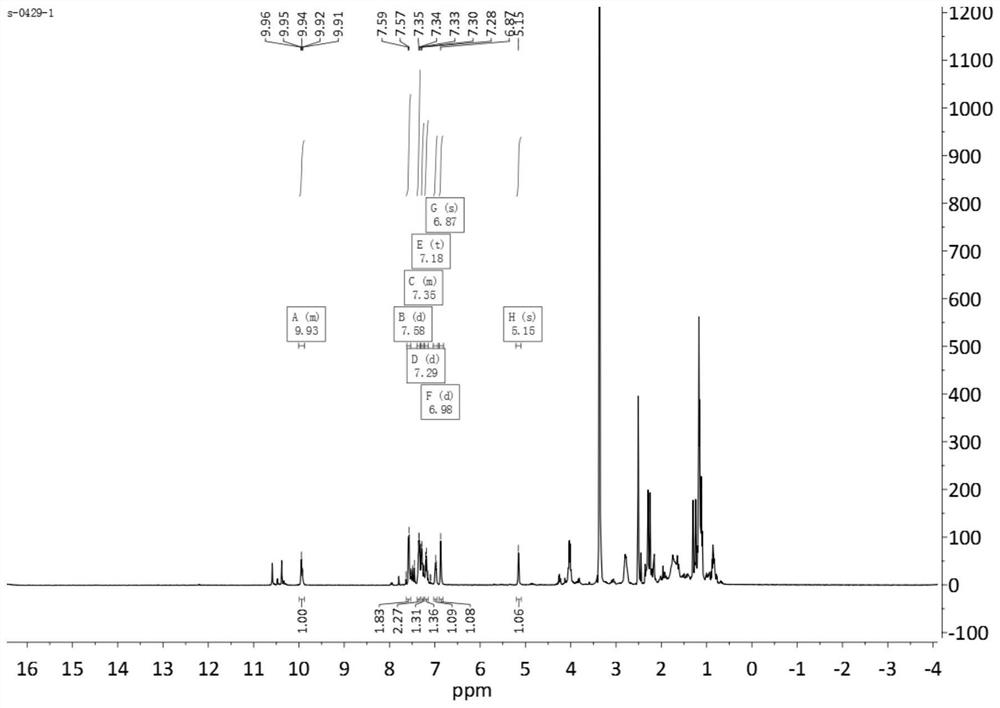
[0049] Add 7mmol of dehydroabietic acid thiosemicarbazide, 7mmol of ethyl acetoacetate, 7mmol of benzaldehyde, 0.7mmol of ytterbium p-trifluoromethanesulfonate, and 20mL of ethanol in sequence in a 50mL eggplant-shaped bottle, heat up to 80°C, and install a reflux device. And react under stirring for 12h, then add 50mL water to the reaction solution to quench; the obtained reaction material is extracted three times with 50mL ethyl acetate, and then washed with water once, the organic phase is combined, the obtained organic phase is rotary evaporated, concentrated, and then Compound a was obtained through silica gel column separation; wherein, during silica gel column chromatography separation, the eluent used was a mixture of petroleum ether and ethyl acetate at a volume ratio of 4:1, and the particle size of the silica gel particles was 100-200 mesh.
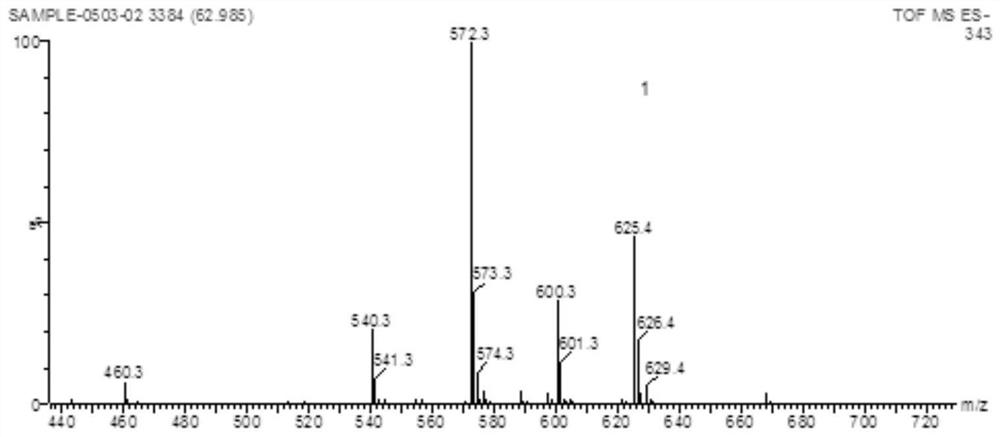
[0050] Yield: 82%, yellow solid (96%); MS (ESI) m / z 572 [M-H]-. 1 HNMR(4...
Embodiment 2
[0052] Preparation of compound b:
[0053]
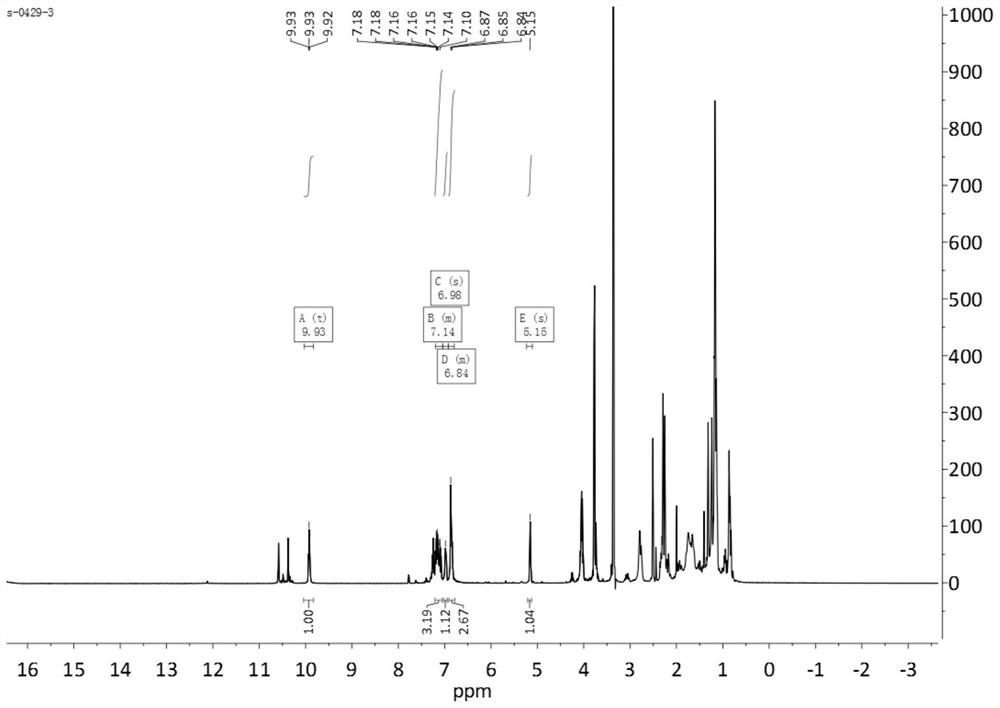
[0054] Add 7mmol of dehydroabietic acid thiosemicarbazide, 7mmol of ethyl acetoacetate, 7mmol of 3-methoxybenzaldehyde, 0.7mmol of ytterbium p-trifluoromethanesulfonate, and 20mL of ethanol in sequence in a 50mL eggplant-shaped bottle, and heat up to 80°C. Install the reflux device, and react under stirring for 12h, then add 50mL water to the reaction liquid to quench; the obtained reaction material is extracted three times with 50mL ethyl acetate, and then washed with water once, the organic phase is combined, and the obtained organic phase is vortexed Evaporation, concentration, and then separated by silica gel column to obtain compound b; wherein, during silica gel column chromatography separation, the eluent used is a mixture of petroleum ether and ethyl acetate with a volume ratio of 4:1, and the particle size of the silica gel particle is 100 -200 mesh.
[0055] Yield: 83%, yellow solid (96%); MS (ESI) m / z 602 [M-H]-. 1 HNM...
Embodiment 3
[0057] Preparation of compound c:
[0058]
[0059] Add 7mmol of dehydroabietic acid thiosemicarbazide, 7mmol of ethyl acetoacetate, 7mmol of 4-bromobenzaldehyde, 0.7mmol of ytterbium p-trifluoromethanesulfonate, and 20mL of ethanol in sequence in a 50mL eggplant-shaped bottle. Reflux device, and react under stirring for 12h, then add 50mL water to the reaction solution to quench; the obtained reaction material is extracted three times with 50mL ethyl acetate, then washed with water once, the organic phase is combined, and the obtained organic phase is subjected to rotary evaporation, Concentrated, and then separated by silica gel column to obtain compound c; wherein, during silica gel column chromatography separation, the eluent used is a mixture of petroleum ether and ethyl acetate with a volume ratio of 4:1, and the particle size of the silica gel particles is 100-200 head.
[0060] Yield: 83%, yellow solid (97%); MS (ESI) m / z 650 [M-H] – . 1 HNMR(400MHz,DMSO)δ10.03–9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com