Preparation method and applications of compound levonorgestrel pessary
A technology of levonorgestrel and vaginal ring, which is applied in the field of pharmacy and can solve problems such as release effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
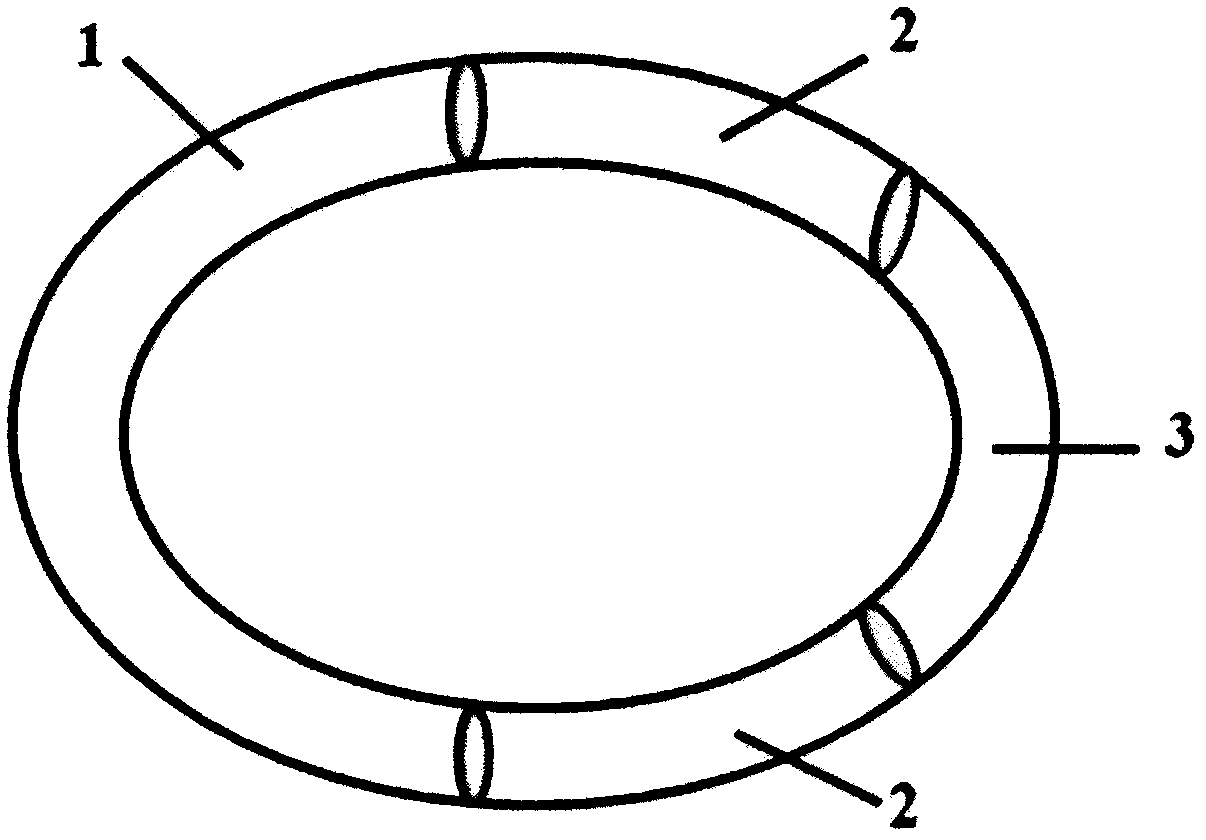
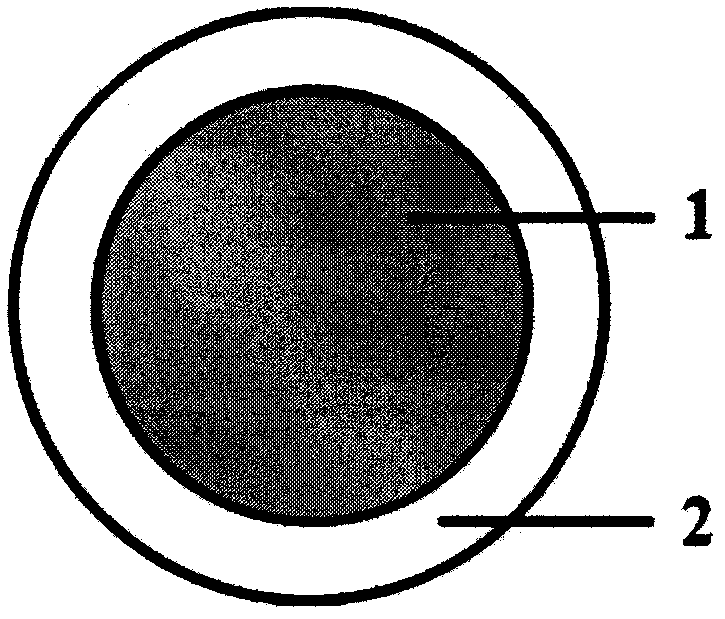
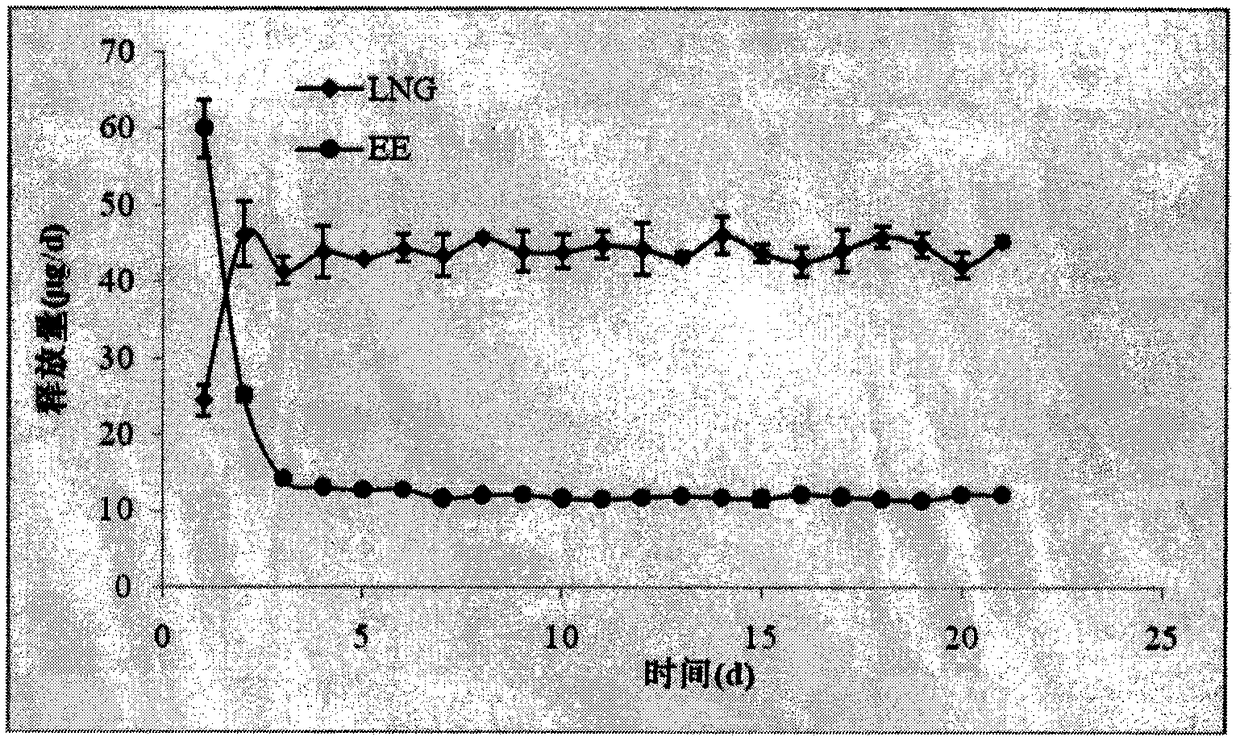
[0028] Accurately weigh 8g of liquid silicone rubber MED-6382 matrix, 0.04g of catalyst, mix for 10min to make it evenly mixed, then weigh 0.2g of levonorgestrel, add it to the silicone rubber, continue to stir for 10min to make it evenly mixed, and then add The mixture is injected into a mold and vulcanized at a high temperature of 80°C for 20 minutes to obtain a drug core loaded with levonorgestrel. The drug core has an outer diameter of 50 mm and a cross-sectional diameter of 4 mm. Accurately weigh 8g of liquid silicone rubber MED-6382 matrix, 0.04g of catalyst, mix for 10min to make it evenly mixed, then weigh 0.048g of ethinyl estradiol, add it to the silicone rubber, continue to stir for 10min to make it evenly mixed, and then inject the mixture In the mold, vulcanize at a high temperature of 80°C for 20 minutes to obtain a drug core loaded with ethinyl estradiol. The drug core has an outer diameter of 50 mm and a cross-sectional diameter of 4 mm. Accurately weigh 15g eac...
Embodiment 2
[0031] Accurately weigh 8g of liquid silicone rubber MED-6382 matrix, 0.04g of catalyst, mix for 10min to make it evenly mixed, then weigh 0.2g of levonorgestrel, add it to the silicone rubber, continue to stir for 10min to make it evenly mixed, and then add The mixture is injected into a mold and vulcanized at a high temperature of 80°C for 20 minutes to obtain a drug core loaded with levonorgestrel. The drug core has an outer diameter of 50 mm and a cross-sectional diameter of 4 mm. Accurately weigh 8g of liquid silicone rubber MED-6382 matrix, 0.04g of catalyst, mix for 10min to make it evenly mixed, then weigh 0.048g of ethinyl estradiol, add it to the silicone rubber, continue to stir for 10min to make it evenly mixed, and then inject the mixture In the mold, vulcanize at a high temperature of 80°C for 20 minutes to obtain a drug core loaded with ethinyl estradiol. The drug core has an outer diameter of 50 mm and a cross-sectional diameter of 4 mm. Accurately weigh 15g eac...
Embodiment 3
[0034] Accurately weigh 4g each of the two components of liquid silicone rubber MED-4950A and B, mix for 10 minutes to make it uniform, then weigh 0.2g of levonorgestrel, add it to the silicone rubber, continue to stir for 10 minutes to make it uniform, then The mixture was injected into a mold, and vulcanized at a high temperature of 80°C for 20 minutes to obtain a drug core loaded with levonorgestrel. The drug core had an outer diameter of 50 mm and a cross-sectional diameter of 4 mm. Accurately weigh 4g each of the two components of liquid silicone rubber MED-4950A and B, mix for 10 minutes to make it uniform, then weigh 0.048g of ethinyl estradiol, add it to the silicone rubber, continue to stir for 10 minutes to make it uniform, and then mix the mixture Inject into a mold and vulcanize at a high temperature of 80°C for 20 minutes to obtain a drug core loaded with ethinyl estradiol. The drug core has an outer diameter of 50 mm and a cross-sectional diameter of 4 mm. Accurat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
| Cross section diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com