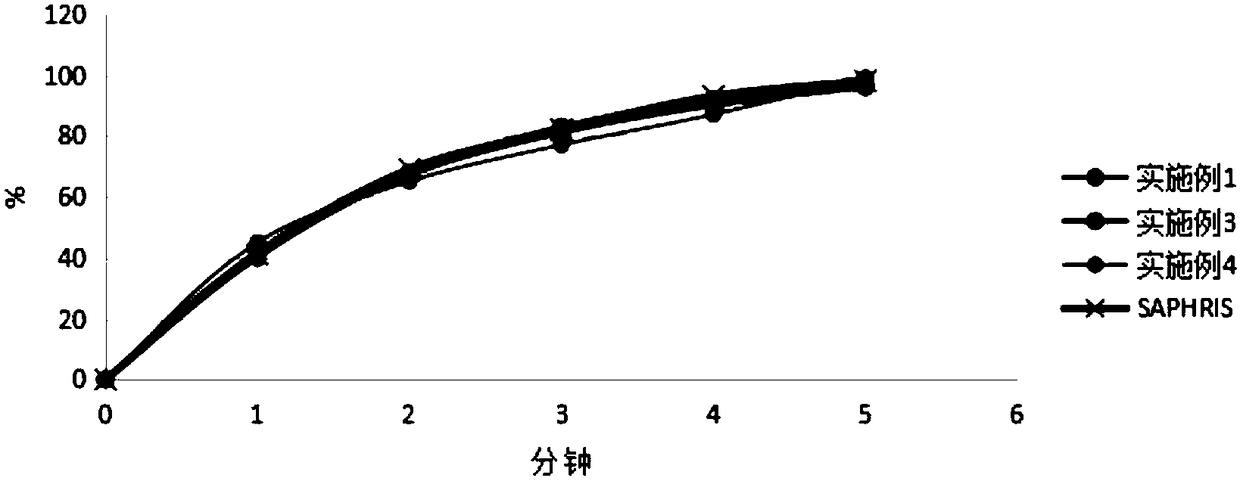
Asenapine maleate tablet and preparation method thereof
A technology of asenapine maleate and excipients, which is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of increasing production cycle and production cost, increasing related substances, Complex formulation process and other problems, to avoid the increase of total impurities, to solve the friability, and to achieve the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
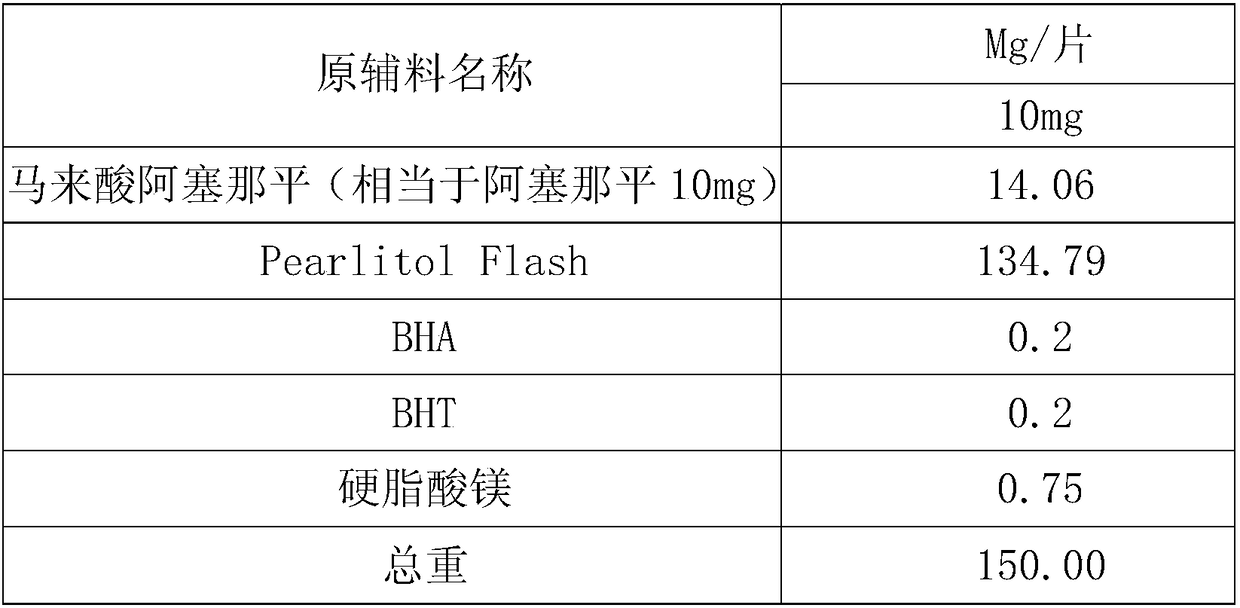
[0027] prescription:
[0028]
[0029] The process steps are as follows:
[0030] 1. Mix asenapine maleate and antioxidant through a 40-mesh sieve;
[0031] 2. After "1" is mixed with the filler / disintegrant compound in equal increments, mix at a medium speed for 10 minutes;
[0032] 3. Pass magnesium stearate through a 40-mesh sieve, add "2" and mix at medium speed for 3 minutes;
[0033] 4. The mixture in "3" is pressed into tablets with a suitable punch (φ7mm), with a hardness of 65-85N.
Embodiment 2
[0035] prescription:
[0036]
[0037] Process steps are as follows:
[0038] 1. Pass asenapine maleate through a 100-mesh sieve and camphor through a 60-mesh sieve for later use;
[0039] 2. First mix the prescribed amount of camphor and PEG6000 evenly, and then mix with the remaining ingredients in the prescription;
[0040] 3. Press the mixed powder directly into tablets, and maintain the hardness at 4-5kg / cm 2 about;
[0041] 4. Heat the tablet at 80°C;
[0042] After 5.5 hours, it was taken out and weighed to confirm that the camphor was completely removed.
Embodiment 3
[0044] prescription:
[0045]
[0046] Process step is with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com