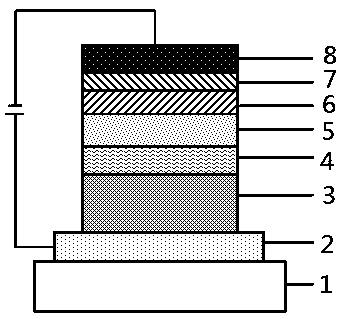
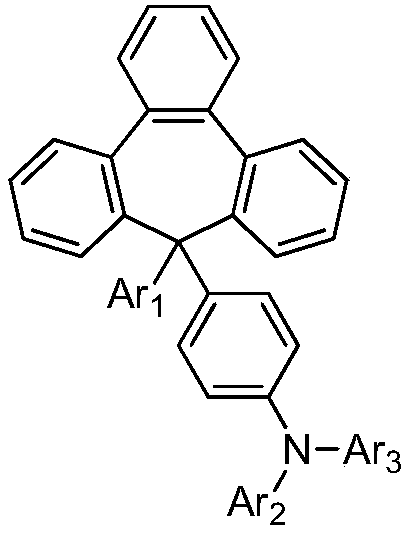
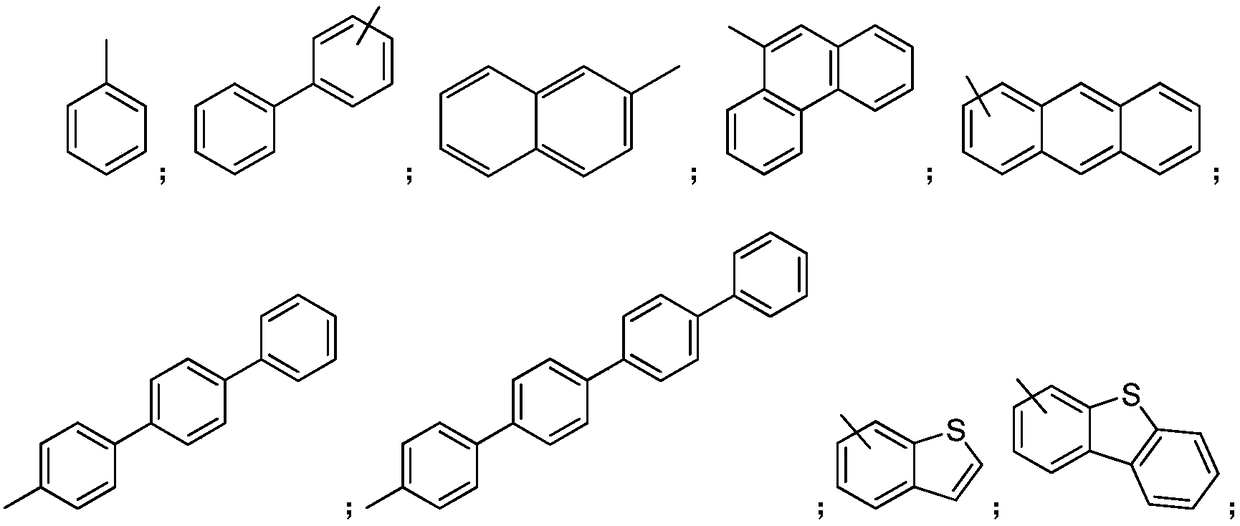
Organic aromatic amine luminescent material containing seven-membered ring
A technology of aromatic amines and luminescent materials, applied in luminescent materials, organic chemistry, electrical components, etc., to achieve good hole injection and hole transport capabilities, improved power efficiency and external quantum efficiency, and reduced driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the synthesis of compound 2
[0031]
[0032] In a 500ml three-necked bottle, add 3.28g of magnesium flakes (134mmol) under the condition of nitrogen gas, at room temperature, prepare raw material A40g (141mol), THF 160g solution, add a small amount of preparation solution to the three-necked bottle, so that the liquid level is just over the magnesium After adding the ingredients, stir and heat up. When the temperature rises to 64°C, the reaction is initiated. When the temperature is lowered to 55°C-65°C, the raw material A / THF solution is added dropwise. Cool down to room temperature, hydrolyze, extract with toluene, wash with water, pass through a silica gel column, remove the solvent, and recrystallize with toluene to obtain 35g of white intermediate A with a yield of 76.5% and a purity of 99.1%. The molar ratio of tribenzocycloheptenone to raw material A is 1.1:1, and the molar ratio of raw material A to magnesium sheet is 1:0.95.
[0033] In a 500...
Embodiment 2
[0035] Embodiment 2: the synthesis of compound 14
[0036]
[0037] Under nitrogen protection, weigh 30g raw material C (177mmol), 37.8g raw material D (177mmol), 42.6g sodium tert-butoxide (443mmol), 0.40g Pd(OAc)2 (1.77mmol), 1.55g C10104R3 (3.54 mmol), stirred and mixed with toluene, heated to 70-75°C, refluxed for 2-3 hours, sampled and spotted on the plate, it showed that there was no raw material C remaining; naturally cooled to room temperature, washed with water, passed through a silica gel column, refined with toluene, and dried to obtain 43g of intermediate B, yield: 80.4%, HPLC: 99.6%. The molar ratio of raw material C, raw material D, and sodium tert-butoxide is 1:1:2.5.
[0038] In a 500ml three-necked flask, under nitrogen gas, add 30g of compound intermediate A (90mmol), 27.1g of intermediate B, add 300g of dichloroethane, and add boron trifluoride ether solution (BF3 Et2O90g, 3 times that of intermediate A), after dropping, keep temperature at 0-10°C, keep...
Embodiment 3
[0040] Embodiment 3: the synthesis of compound 47
[0041]
[0042] Under nitrogen protection, weigh 30g raw material C (177mmol), 70.5g raw material E (177mmol), 42.6g sodium tert-butoxide (443mmol), 0.40g Pd(OAc)2 (1.77mmol), 1.55g C10104R3 (3.54 mmol), stirred and mixed with toluene, heated to 70-75°C, refluxed for 3-5 hours, sampling TLC showed that no raw material C remained; naturally cooled to room temperature, washed with water, passed through a silica gel column, refined with toluene, and dried to obtain 55g Intermediate C, yield: 63.9%, HPLC: 99.8%. The molar ratio of raw material C, raw material E, and sodium tert-butoxide is 1:1:2.5.
[0043]In a 500ml three-neck flask, under nitrogen gas, add 30g of compound intermediate A (90mmol), 43.6g of intermediate C (90mmol), add 300g of dichloroethane, and add boron trifluoride ether dropwise at a temperature of 0-10°C Solution (BF 3 ·Et 2 (2) 150g, 5 times that of intermediate A), after dripping, temperature contro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com