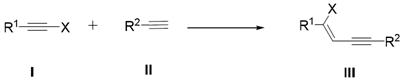
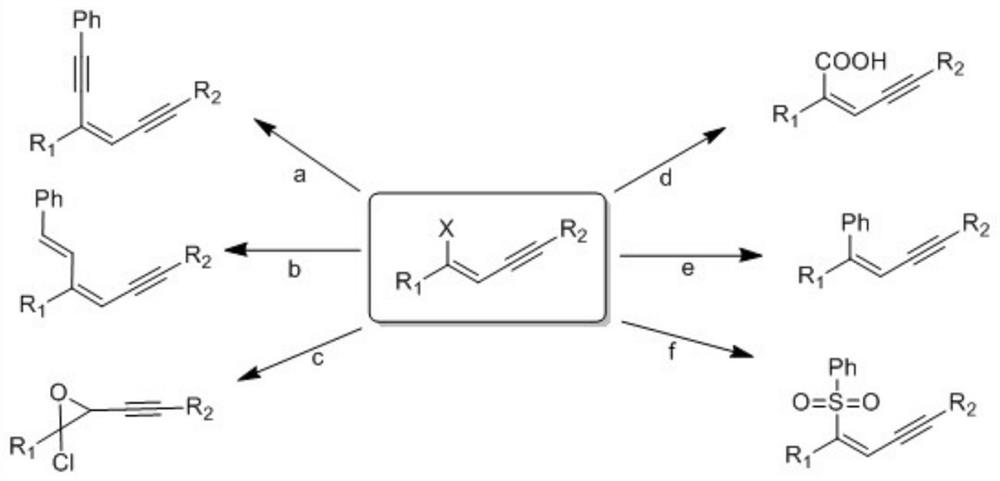
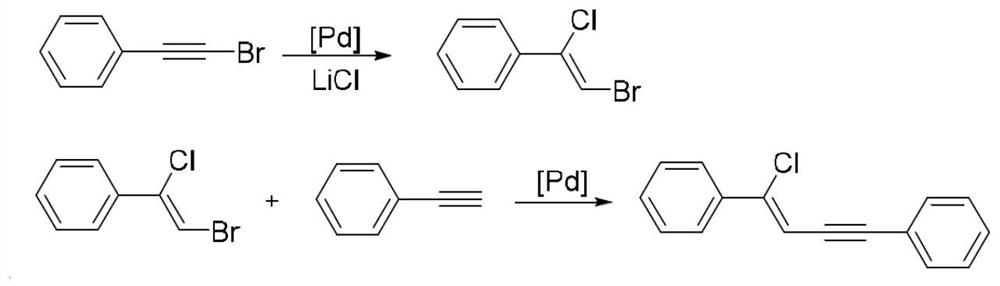
Halogenated conjugated 1,3-enyne compound and its preparation method and application
A compound and conjugation technology, applied in halogenated conjugation 1, can solve the problems of few alternative methods and complex synthetic pathways of halogenated conjugated enynes, and achieve simple and convenient methods, good inhibition selectivity, and wide application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of alkyne halide (I)
[0035] (1) Preparation of phenyl alkyne chloride (I-1)
[0036] The reaction formula is as follows:
[0037]
[0038] Add phenylacetylene (II-1) (2.042g, 20mmol), cesium carbonate (3.84g, 22mmol), tetra-n-butylammonium chloride (278mg, 1mmol) into a round-bottomed flask of carbon tetrachloride, at 70 The reaction was stirred at °C for 7 hours. After the reaction, add n-hexane (100 mL) to dilute, filter, collect the filtrate, use a rotary evaporator to evaporate the solvent under reduced pressure in a water bath at 40°C. Finally, column chromatography (mobile phase is petroleum ether) yielded a colorless target compound (I-1) with a yield of 83.0%. Its structure is characterized as follows:
[0039] 1 H NMR (600MHz, CDCl 3 )δ7.36-7.33(m,2H),7.24-7.20(m,3H). 13 C NMR (150MHz, CDCl 3 )δ131.9,128.6,128.3,122.1,69.4,68.0.GC-MS(EI):m / z 136[M + ],138[(M+2) + ].
[0040] The preparation methods of other substitu...
Embodiment 2
[0053] Example 2: Preparation of chloroconjugated 1,3-enynes (III-1)
[0054] The reaction formula is as follows:
[0055]
[0056] Add IPrAuCl (5mol%, 9.3mg, 0.015mmol) in reaction bottle, NaBArF 4 (10mol%, 26.6.3mg, 0.03mmol), first dissolve with 2.0mL dichloroethane, add phenylacetylene (II-1) (30.6mg, 0.3mmol) in the reaction flask at 40°C, start stirring, and add Add phenylalkyne chloride (I-1) (41.0 mg, 0.3 mmol) and stir for 2 hours, and monitor the reaction process with a TLC plate. After the reaction is finished, filter and wash the filter cake with dichloromethane (0.6mL×2), combine the filtrates, evaporate the solvent, and finally purify by column chromatography (mobile phase is petroleum ether or n-hexane, Rf=0.6) to obtain chlorine Substituting conjugated 1,3-enynes (III-1) with a yield of 85%. Its structure is characterized as follows:
[0057] 1 H NMR (600MHz, CDCl 3 )δ7.66(dd, J=7.8,1.8Hz,2H),7.53(dd,J=6.7,3.0Hz,2H),7.40-7.37(m,3H),7.34(dd,J=7.0,2.8Hz ...
Embodiment 3
[0058] Example 3: Preparation of halogenated conjugated 1,3-enynes (III-2)
[0059] The reaction formula is as follows:
[0060]
[0061] Phenylacetylene (II-1) (30.6mg, 0.3mmol) in Example 2 was replaced by p-fluorophenylacetylene (II-2) (33.0mg, 0.3mmol), other operations were the same as in Example 2, and finally chlorine Substituting conjugated 1,3-enynes (III-2) with a yield of 83%. Its structure is characterized as follows:
[0062] 1 H NMR (600MHz, CDCl 3 )δ7.53(d, J=8.1Hz, 2H), 7.49(dd, J=8.3,5.6Hz, 2H), 7.17(d, J=8.1Hz, 2H), 7.02(t, J=8.5Hz, 2H), 6.38(s, 1H), 2.36(s, 3H). 13 C NMR (150MHz, CDCl 3 )δ162.72 (d, J=249), 142.76, 139.98, 133.79, 133.60 (d, J=7.5), 129.28, 126.22, 119.34 (d, J=18), 115.73 (d, J=22.5), 105.73 ,94.48,86.04,21.27.IRν max (cm -1 ):3415,1593,1496,1388,1225,823,803; GC-MS(EI):m / z256[M + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com