Method for preparing pesticide intermediate 2,6-diethyl-4-methylphenyl bromide and method for recovering waste hydrobromic acid
A technology of methyl bromide and waste hydrobromic acid, applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, organic chemistry, etc., can solve the problems of waste hydrobromic acid pollution, reduce production costs, etc., and achieve pollution, reduce The effect of reducing production cost and raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
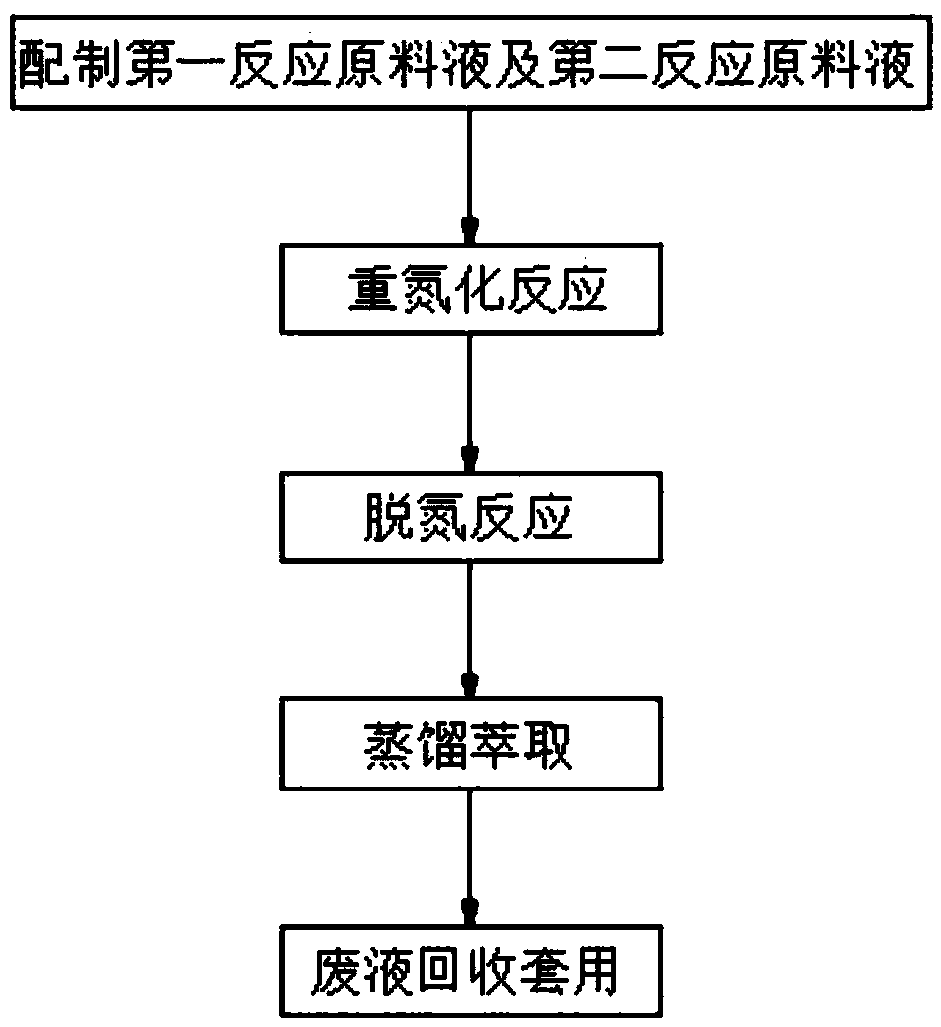
[0033] The invention discloses a preparation method of a pesticide intermediate 2,6-diethyl-4-methylbromobenzene, specifically discloses a preparation process of 2,6-diethyl-4-methylbromobenzene, comprising the following step:
[0034] S1, in the reactor that has stirrer, thermometer and constant pressure dropping funnel, add the 2 of 0.2mol successively, the 40% hydrobromic acid of 6-diethyl-4-methylaniline and 100ml, stir and be heated to 80~90℃, react for 15-20 minutes, and obtain a white suspension;
[0035] S2, cooling the above white suspension to -10°C;
[0036] S3. Prepare a mixed solution of sodium nitrite and water, and control the temperature of the mixed solution at -5 to 0°C, wherein, 0.24 mol of sodium nitrite and 50 mL of water are slowly added dropwise to the above white suspension solution, after the dropwise addition, a yellow suspension was obtained;
[0037] S4. Add 160 mg of sulfamic acid or 500 mg of urea to the yellow suspension, continue to stir for ...
Embodiment 2
[0049] The invention discloses a preparation method of a pesticide intermediate 2,6-diethyl-4-methylbromobenzene, specifically discloses a preparation process of 2,6-diethyl-4-methylbromobenzene, comprising the following step:
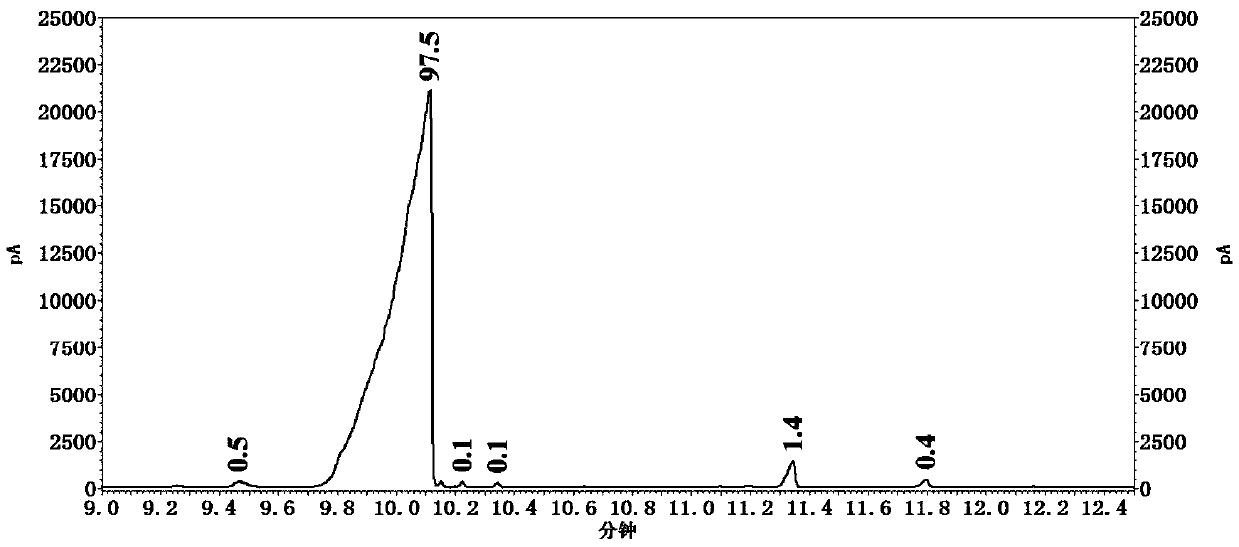
[0050] S1, in the reactor that has stirrer, thermometer and constant pressure dropping funnel, add the 2 of 0.2mol successively, the 41.79% hydrobromic acid of 6-diethyl-4-methylaniline and 100ml, stir and be heated to 80~90 DEG C, reaction 15-20 minute, obtain white suspension liquid, wherein, 41.79% hydrobromic acid comes from the waste hydrobromic acid recovered when preparing 2,6-diethyl-4-methylbromobenzene in Example 1 ;
[0051] S2. Cool the above white suspension to -15°C;
[0052] S3. Prepare a mixed solution of sodium nitrite and water, and control the temperature of the mixed solution at -5 to 0°C, wherein, 0.24 mol of sodium nitrite and 50 mL of water are slowly added dropwise to the above white suspension solution, after the dropwise addi...
Embodiment 3
[0065] The invention discloses a preparation method of a pesticide intermediate 2,6-diethyl-4-methylbromobenzene, specifically discloses a preparation process of 2,6-diethyl-4-methylbromobenzene, comprising the following step:
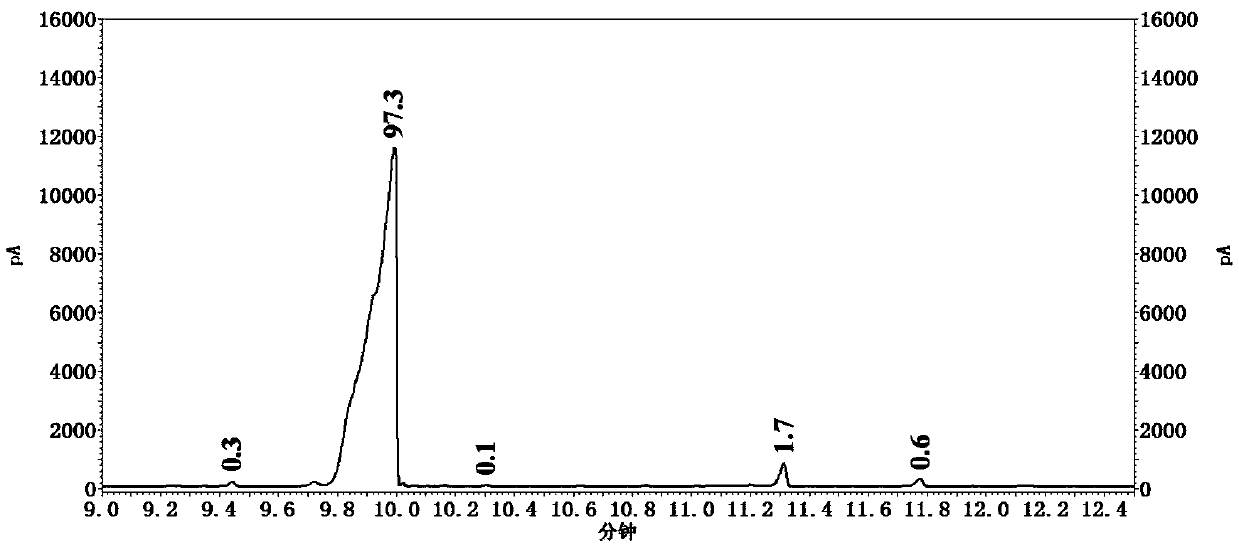
[0066] S1, in the reactor that has stirrer, thermometer and constant pressure dropping funnel, add the 2 of 0.2mol successively, the 41.03% hydrobromic acid of 6-diethyl-4-methylaniline and 100ml, stir and be heated to 80~90 ℃, reaction 15-20 minute, obtain white suspension liquid, wherein, 41.03% hydrobromic acid comes from the waste hydrobromic acid recovered when preparing 2,6-diethyl-4-methylbromobenzene in Example 2 ;
[0067] S2. Cool the above white suspension to -12°C;
[0068] S3. Prepare a mixed solution of sodium nitrite and water, and control the temperature of the mixed solution at -5 to 0°C, wherein, 0.24 mol of sodium nitrite and 50 mL of water are slowly added dropwise to the above white suspension solution, after the dropwise additi...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap