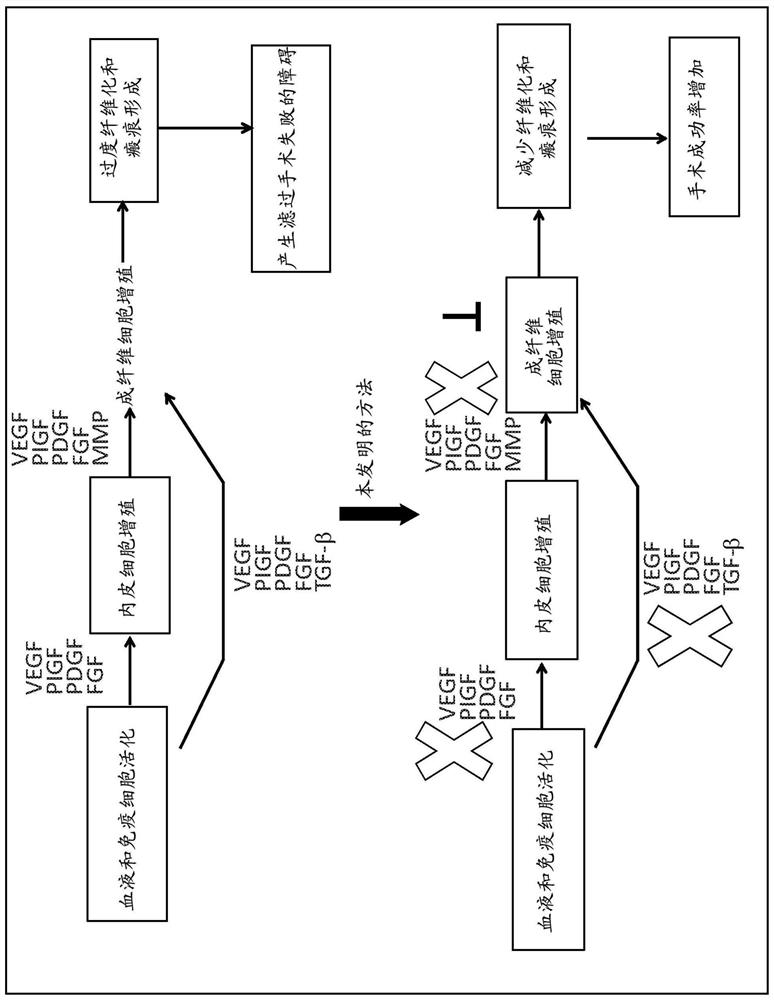
Compositions and methods for improving the success of glaucoma surgery using nintedanib
A technology for nintedanib and glaucoma, applied in the field of improving the success rate of glaucoma surgery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
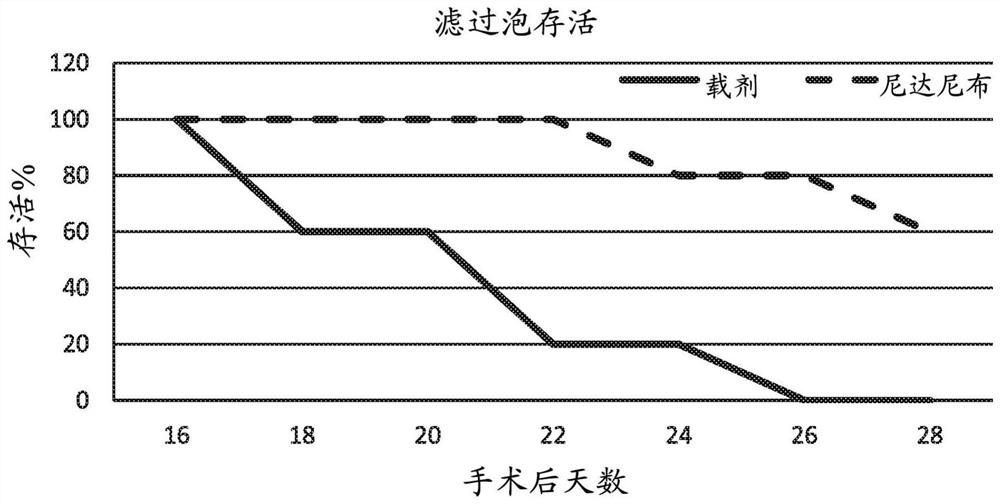
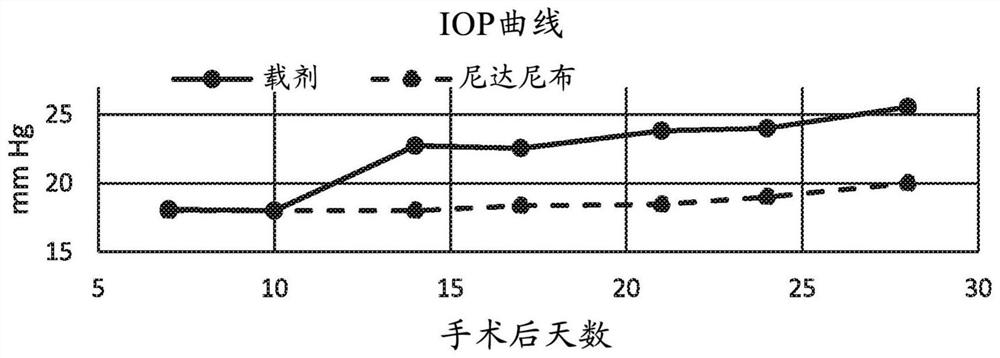
[0050] Embodiment 1: Rabbit glaucoma surgery model
[0051]A rabbit model of glaucoma surgery was used to demonstrate the success of glaucoma filtering surgery using the methods disclosed in the present invention. In particular, the established glaucoma filtration surgery model in rabbits was used to study the effect of 0.2% nintedanib solution on postoperative wound healing events. Surgical procedures were as described by Wong et al. (Wong et al., Invest Ophthalmol Vis Sci. 2003;44(3):1097-1103). Briefly, a partially torn 8-0 silk corneal traction suture was placed over and the eye was pulled down. The fornix-based conjunctival flap was lifted, after which blunt dissection of the subconjunctival space was performed approximately 5 mm along the limbus, and 8 mm posteriorly. A partially torn scleral incision was made 3 to 4 mm posterior to the limbus using a microretinal knife (MVR) and a scleral tunnel to the corneal stroma was created. A 22-gauge, 25-mm intravenous cannula...
Embodiment 2
[0056] Example 2: Nintedanib Topical Ophthalmic Preparation as Adjuvant Therapy for Glaucoma Filtration Surgery
[0057] In a clinical study, topical nintedanib 0.2% was administered as adjuvant therapy to increase the success of trabeculectomy. The effect of topical nintedanib 0.2% on the success rate of trabeculectomy was investigated in a randomized, double-blind, placebo-controlled, 12-month trial. The study design was as described by Vandewalle et al. (Vandewalle et al., Br J Ophthalmol. 2014, Jan;98(1):73-8).
[0058] Patients with medically uncontrolled open-angle glaucoma undergoing planned primary trabeculectomy will be enrolled and randomized to receive one drop of nintedanib three times daily or a placebo solution. Treatment starts immediately after surgery and will continue for 1 month. Approximately 150 patients were enrolled in this study.
[0059] Surgery will be performed by an experienced surgeon under general or retrobulbar anesthesia using a modified Moor...
Embodiment 3
[0064] Example 3: Formulation
[0065] Nintedanib Ophthalmic Solution
[0066] The drug product is an isotonic ophthalmic solution prepared in 2-hydroxypropyl beta-cyclodextrin or other similar cyclodextrin and a buffered solution (pH range from 5.5 to 8.0). Other viscosity builders, lubricants, preservatives may be added to enhance the functionality of the formulation. See Table 1 for the composition of the ophthalmic solution.
[0067] Table 1 Nintedanib Ophthalmic Solution
[0068]
[0069]
[0070] Nintedanib Ophthalmic Suspension
[0071] The drug product is an isotonic ophthalmic suspension prepared in sodium carboxymethylcellulose and buffer solution (pH range from 5.5 to 8.0). The drug particle size is reduced to less than 40 microns. Other viscosifiers, lubricants, solubilizers and preservatives may be added to enhance the functionality of the formulation suspension. The composition is shown in Table 2.
[0072] Table 2 Nintedanib Ophthalmic Suspension
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com