Hemostasis composition including cross-linking hyaluronic acid derivative matrix
A technology of hyaluronic acid and its derivatives, which can be used in drug combinations, medical preparations containing active ingredients, and blood diseases, etc. It can solve problems such as low viscosity and hygroscopicity, damage to wound areas, and mixing of ingredients that are difficult to stop bleeding. Adhesion, the effect of excluding the risk of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] As another aspect, the present invention relates to a method for the preparation of a hemostatic composition comprising a cross-linked hyaluronic acid derivative matrix. Specifically, the preparation method may include: i) a step of reacting hyaluronic acid and an epoxy cross-linking agent, wherein the hyaluronic acid and the epoxy cross-linking agent are dissolved in an alkaline aqueous solution to prepare a cross-linked transparent a hyaluronic acid derivative; and ii) a step of homogenizing the cross-linked hyaluronic acid derivative to prepare it to an appropriate size.
[0037] Preferably, in the preparation method of hyaluronic acid derivatives according to the present invention, the hyaluronic acid (HA) obtained by reacting an epoxy crosslinking agent containing two or more epoxy groups with hyaluronic acid (HA) Hyaluronic acid derivatives obtained by product homogenization.
[0038] In the preparation method of the present invention, hyaluronic acid (HA) or its...
Embodiment 1-3
[0056] Example 1-3. Preparation of Hyaluronic Acid Derivatives of Hemostatic Composition
[0057] a. Preparation of cross-linked hyaluronic acid derivative matrices
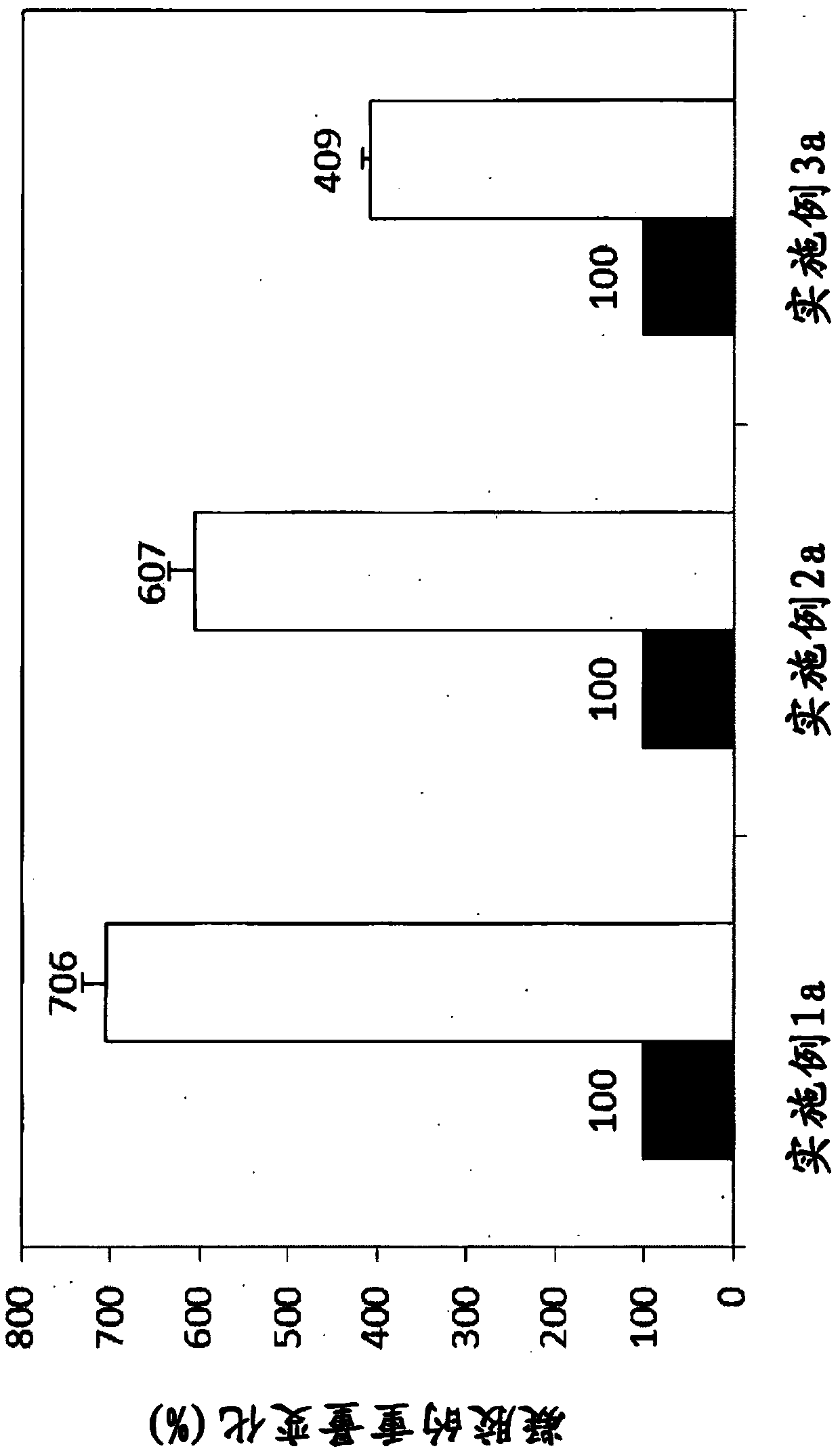
[0058] After preparing 1 g of sodium hyaluronate in each of the 3 reactors, it was added to a final weight of 10.0 g (Example 1a), 8.3 g (Example 2a) and 7.1 g (Example 2a) using 0.25 N NaOH solution. Example 3a). 70 μL (Example 1a), 60 μL (Example 2a) and 50 μL (Example 3a) of 1,4-butanediol diglycidyl ether (BDDE) were added to the completely dissolved solution, and then they were mixed. The mixed solution was placed in a constant temperature water bath and reacted at 30° C. for 18 hours, and then washed with a buffer solution to remove unreacted substances. The prepared gel was homogenized 3 times or more by the compression method to control the particle size, and then it was sterilized at 121° C. for 15 minutes. 3.0 g of the prepared hyaluronic acid derivatives were aseptically weighed in a 5 ml syring...
Embodiment 4
[0063] Example 4. Rheological Properties of Hyaluronic Acid Derivatives
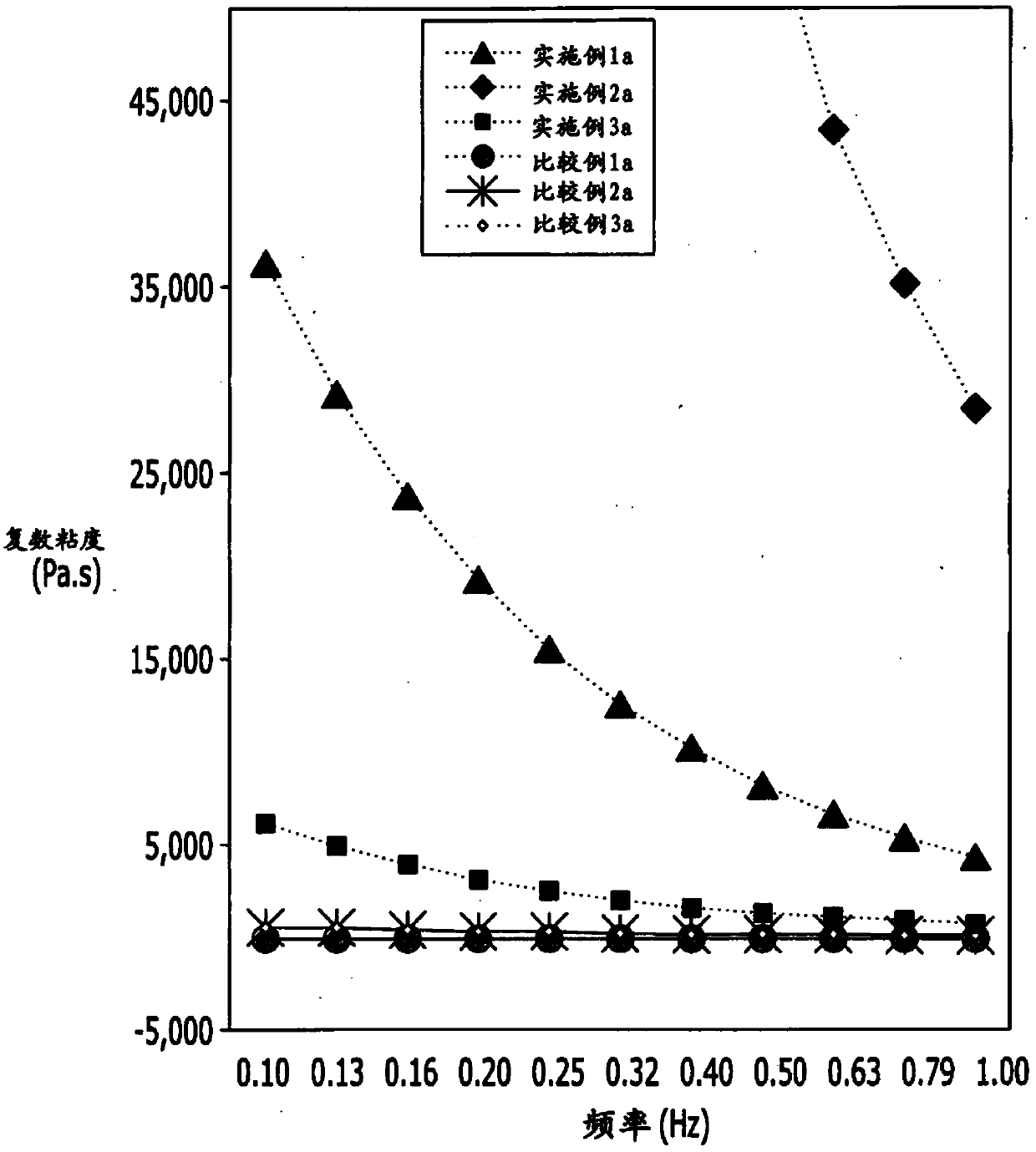
[0064]In order to study the hyaluronic acid derivatives prepared as in Examples 1a-3a and the commercially available anti-adhesive agent of company B (comparative example 1a) containing hyaluronic acid and the cross-linked hyaluronic acid filler of company G (Comparative Example 2a) and the rheological properties of the cross-linked hyaluronic acid filler (Comparative Example 3a) of L Company were tested by rotational rheometer. The complex viscosity and tanδ results in the frequency range of 0.1Hz to 1Hz are shown in figure 2 .
[0065] pass figure 2 and Table 1, Examples 1a to 3a show higher complex viscosity values compared to Comparative Examples 1a to 3a. From this, it can be seen that the hyaluronic acid derivatives of the present invention (Examples 1a to 3a) have higher viscosities compared with Comparative Examples 1a to 3a, and they form structures with high structural stability.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| complex viscosity | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com