Digital biomarkers for cognition and movement diseases or disorders
A technology for sports diseases and disorders, applied in the field of diagnosis, which can solve time-consuming and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0442] Example 1: Computer-implemented (electronic) Symbolic Digital Modal Testing (eSDMT)
[0443] A smartphone with a 5.1-inch screen is programmed with the kit for performing the eSDMT test. The tester is asked to perform the test on the smartphone according to the instructions shown on the display. 30 subjects were investigated. The determined response and accuracy are shown in the Figure 4 middle.
[0444] The time elapsed between subsequent response (R) and subsequent correct response (CR) was also investigated in the implemented eSDMT test. The results are shown in Figure 5 middle.
[0445] In addition, response (R) and correct response (CR) profiles were determined. Examples of Response (R) and Correct Response (CR) profiles of two subjects with distinctly different performances on the eSDMT test are shown in Figure 6 middle.
example 2
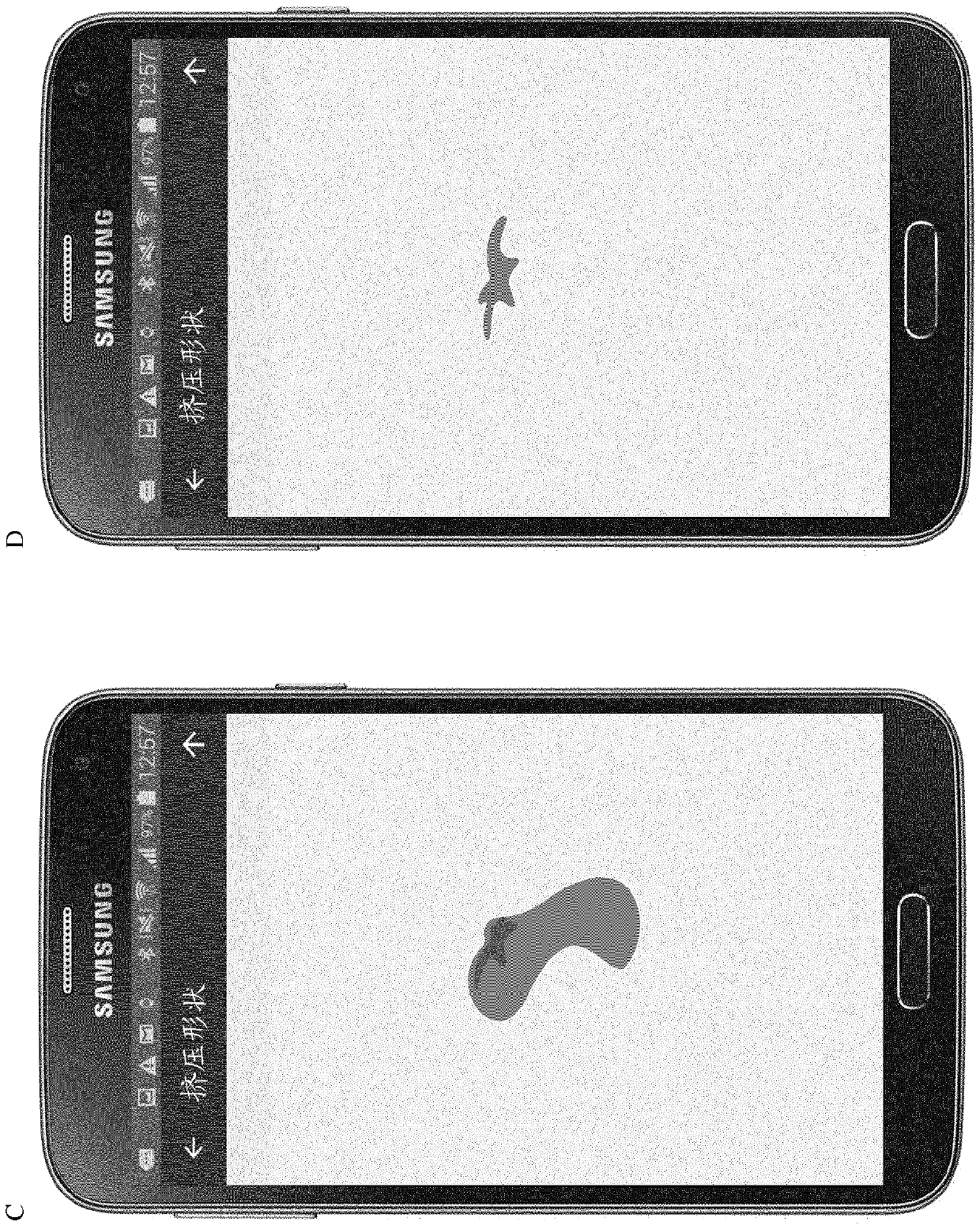
[0446] Example 2: Computer-implemented test to assess fine motor skills (fine motor assessment), particularly hand motor function, and specifically the touchscreen-based "draw shape" and "squeeze shape" tests
[0447] A smartphone with a 5.1-inch screen was programmed with a suite for performing the "draw shape" and "squeeze shape" tests. The tester is asked to perform the test on the smartphone according to the instructions shown on the display.
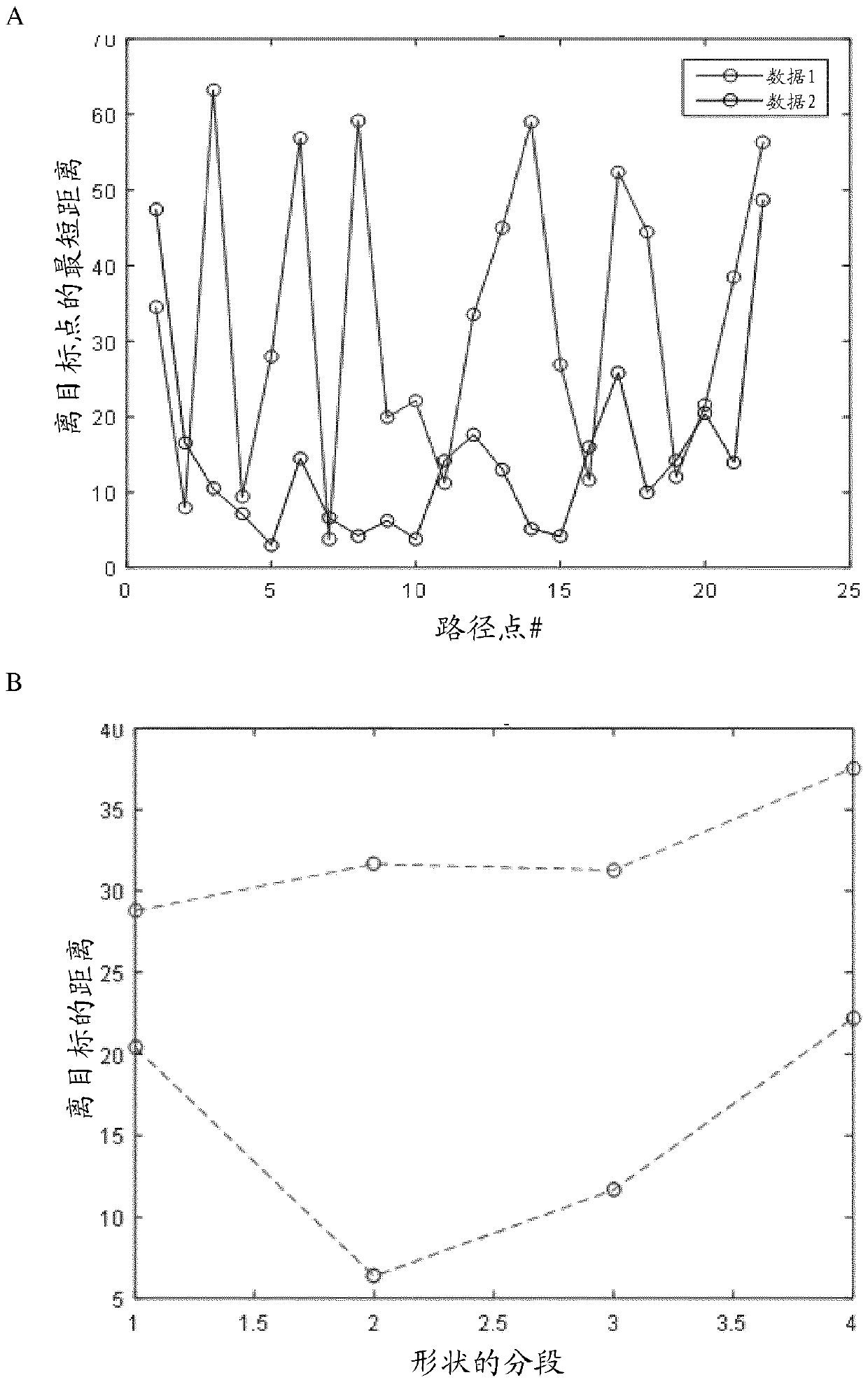
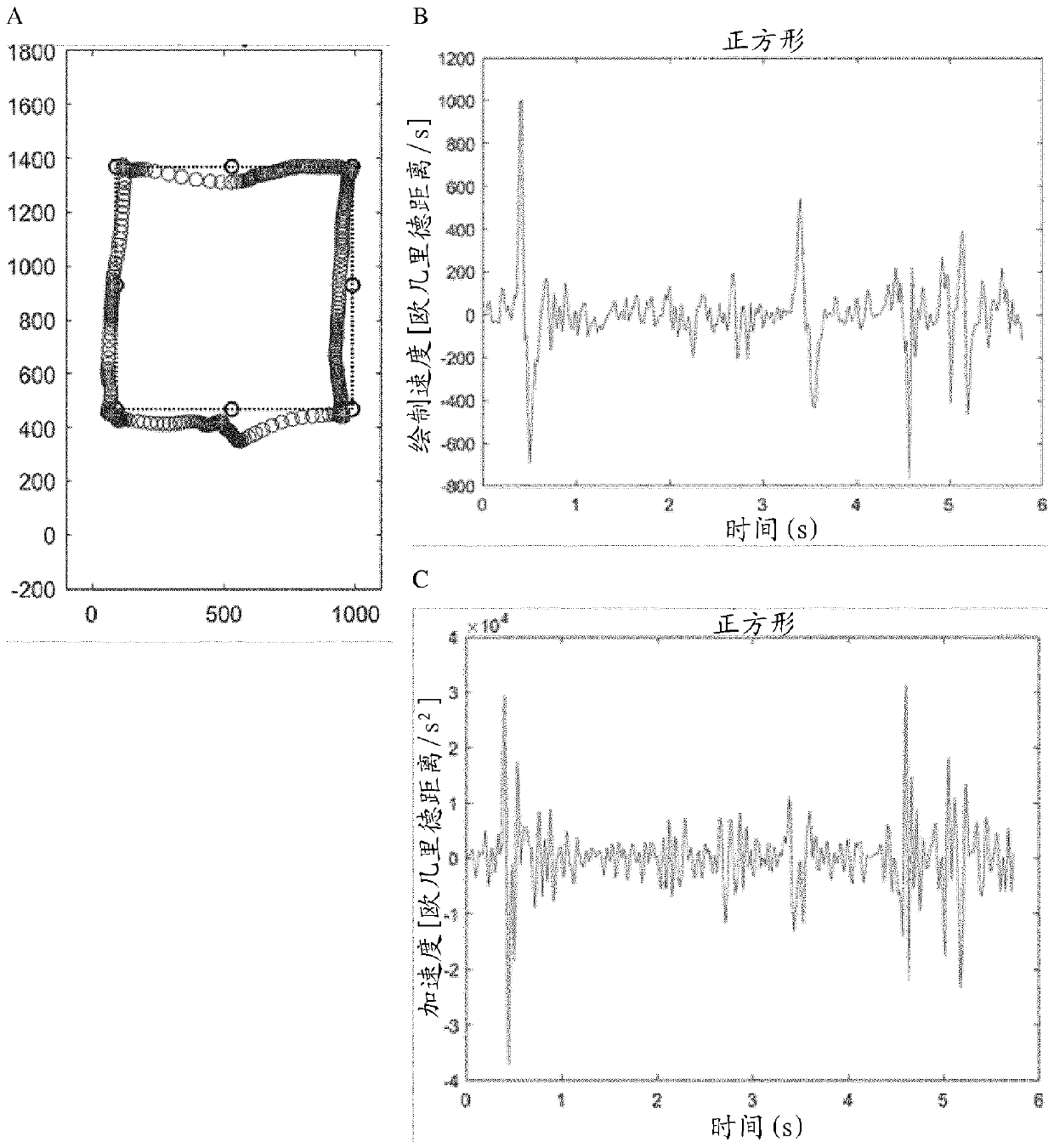
[0448] In the pinch shape setup, touch events from the first and second fingers are determined and the distance and velocity of the pinch events are calculated ( Figure 8 ). In the drawing shape setting, a circle-shaped touch trace is defined. The results are plotted in Figure 8 or Figure 10 middle.
[0449] The overall calculated trace performance is shown separately in Figure 9 and Figure 11 , and detailed data are summarized in Table 1 or Table 2 below.
[0450] Table 1: Circle evaluation readout performance statisti...
example 3
[0455] Example 3: Results from a prospective pilot study (FLOODLIGHT) to evaluate the feasibility of using digital technologies for remote patient monitoring in patients with multiple sclerosis
[0456] Study populations will be selected by using the following inclusion and exclusion criteria:
[0457] Key inclusion criteria:
[0458] Sign the informed consent
[0459] Able to comply with the study protocol, in the investigator's judgment
[0460] Age 18-55, including 18 and 55
[0461] Diagnosed with MS, confirmed according to revised McDonald 2010 guidelines
[0462] EDSS score from 0.0 to 5.5 inclusive
[0463] Weight: 45-110kg
[0464] For women of reproductive potential: Agree to use an acceptable method of contraception during the study period
[0465] Key strikeout guidelines:
[0466] Patients who, in the judgment of the investigator, are seriously ill and unstable
[0467] Change dosing regimen or switch disease-modifying therapy (DMT) within the last 12 weeks...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com