Cysteine engineered fibronectin type iii domain binding molecules
A technology of cysteine and fibronectin, applied in the direction of peptide/protein components, animal/human proteins, anti-animal/human immunoglobulins, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0365] Example 1: Construction of Tencon Library
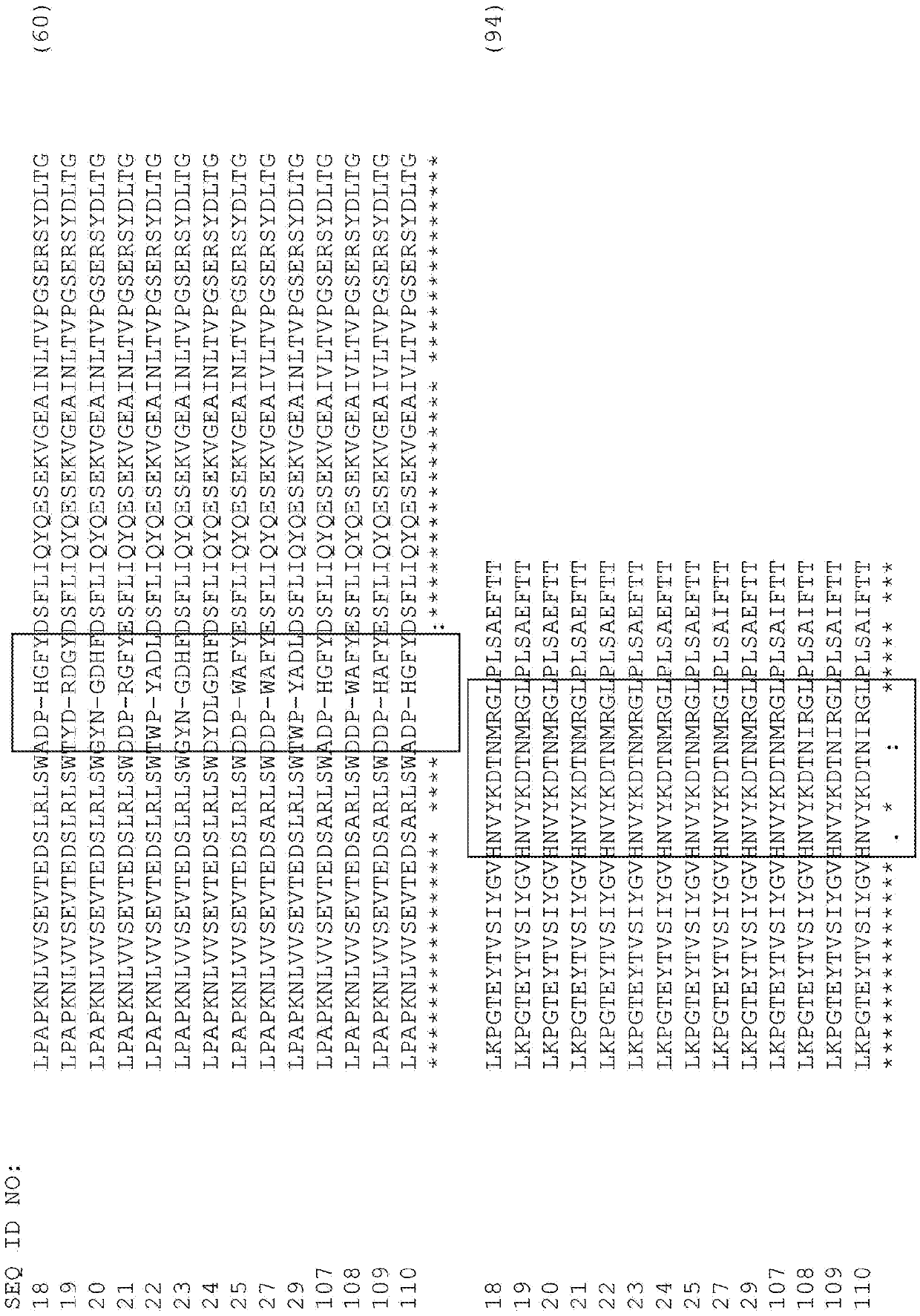
[0366] Tencon (SEQ ID NO:1) is an immunoglobulin-like scaffold type III fibronectin (FN3) domain designed from the consensus sequence of fifteen FN3 domains of human tenascin-C (Jacobs et al., Protein Engineering, Design, and Selection, 25:107-117, 2012; US Patent Publication 2010 / 0216708). The crystal structure of Tencon shows six surface exposed loops connecting seven β-strands. The selected residues within these loops or each loop can be randomized to construct a type III fibronectin (FN3) domain library that can be used to select novel molecules that bind to specific targets.
[0367] Tencon:
[0368] Lpapknlvvsevtedslrlswtapdaafdsfliqyqesekvgeainltvpgsersydltglkpgteytvsiygvkgghrsnplsaeftt (SEQ ID NO 1):
[0369] TCL1 library construction
[0370] The library TCL1 designed to randomize only the FG loop of Tencon (SEQ ID NO:1) was constructed for use with the cis-display system (Jacobs et al., Protein Engineering, Design, and Sele...
Embodiment 2
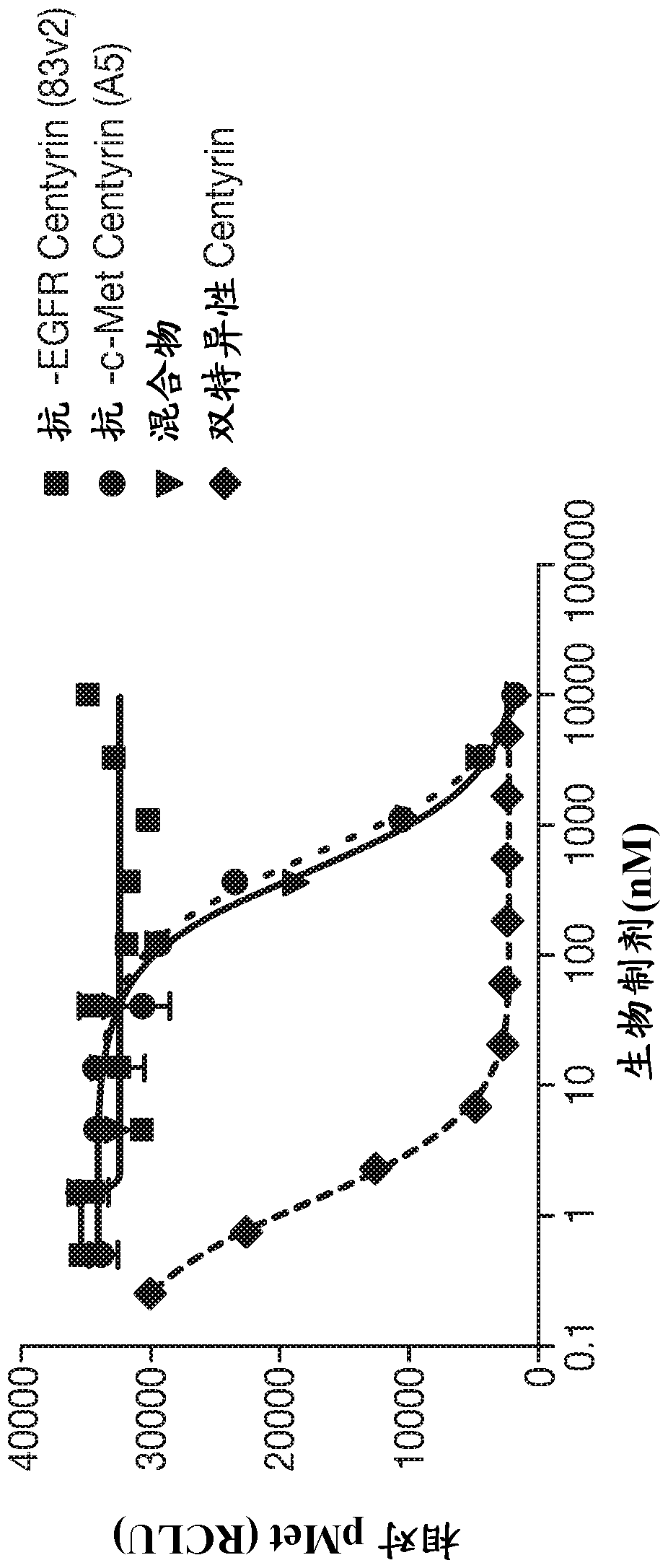
[0388] Example 2: Selection of type III fibronectin (FN3) domain that binds EGFR and inhibits EGF binding
[0389] Library screening
[0390] The cis display was used to select EGFR binding domains from TCL1 and TCL2 libraries. The recombinant human extracellular domain of EGFR fused to IgG1 Fc (R&D Systems) was biotinylated using standard methods and used for panning (residues 25-645 of the full-length EGFR of SEQ ID NO: 73). For in vitro transcription and translation (ITT), 2-6 µg of library DNA are incubated with 0.1 mM intact amino acids, 1X S30 premix fraction and 30 µL S30 extract (Promega) in a total volume of 100 µL and incubated at 30°C . After 1 hour, 450 μL of blocking solution (PBS pH 7.4, supplemented with 2% bovine serum albumin, 100 μg / mL herring sperm DNA, and 1 mg / mL heparin) was added, and the reaction was incubated on ice for 15 minutes. EGFR-Fc was assembled by mixing recombinant human EGF (R&D Systems) and biotinylated recombinant EGFR-Fc in blocking solutio...
Embodiment 3
[0408] Example 3: Characterization of EGFR-binding FN3 domain that inhibits EGF binding
[0409] Large-scale expression, purification and endotoxin removal
[0410] The 9 FN3 domains shown in Table 4 are enlarged to provide more materials for detailed characterization. Using an overnight culture containing each EGFR-binding FN3 domain variant, the overnight culture was diluted 1 / 80 in 0.8 L Terrific broth supplemented with 100 µg / mL ampicillin and inoculated into fresh medium, Incubate with shaking at 37°C. When the optical density at 600 nm reached about 1.2-1.5 by adding IPTG to a final concentration of 1 mM, the culture was induced and the temperature was lowered to 30°C. After 4 hours, the cells were collected by centrifugation, and the cell pellets were stored at -80°C until needed.
[0411] For cell lysis, the thawed pellets were thawed at 5mL BugBuster per gram of pellets ® Resuspended in supplement with 25U / mL Benzonase ® (Sigma-Aldrich) and 1kU / mL rLysozyme ™ (Novagen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com