Copolyester containing furan ring and its preparation method
A technology of copolyester and furan ring, applied in the field of furan ring-containing copolyester and its preparation, can solve the problems of low tensile strength, deep color in reaction time, low tensile modulus and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of the copolyester containing furan ring provided by the invention comprises the following steps:
[0032] (1) The first component, the second component, the third component and the fourth component are provided, wherein the first component includes at least one of furandicarboxylic acid and furandicarboxylic acid ester, so The second component includes at least one of aromatic diols and aliphatic diols, the third component includes polyhydric alcohols with a hydroxyl number greater than or equal to 3, and the fourth component includes a carbonyl number greater than or equal to 3 anhydrides;
[0033] (2) Mix the first component, the second component, the third component, the fourth component and the esterification catalyst, and react under an inert atmosphere or a nitrogen atmosphere to obtain the first Intermediate product, wherein the molar ratio of the first component, the second component, the third component and the fourth component is 1:(1....
Embodiment 1
[0079] Add dimethyl 2,5-furandicarboxylate, ethylene glycol, pyromellitic anhydride, pentaerythritol and anhydrous zinc acetate into the reaction kettle at a molar ratio of 1:1.6:0.003:0.0005:0.001, and under an inert atmosphere , gradually warming up to 185°C, and reacting for 4.5h. Then add 0.12% antimony trioxide, 0.2% triphenyl phosphate, and 0.1% antioxidant-1010 in the molar weight of dimethyl 2,5-furandicarboxylate, and slowly evacuate to 600Pa~ 2000Pa, pre-polymerized at 220°C for 0.5h. Then gradually raise the temperature to 240° C., continue vacuuming to below 200 Pa and react for 3 hours to obtain a furan ring-containing copolyester.
[0080] from figure 1 It can be seen that the furan ring-containing copolyester obtained in this example is very light yellow.
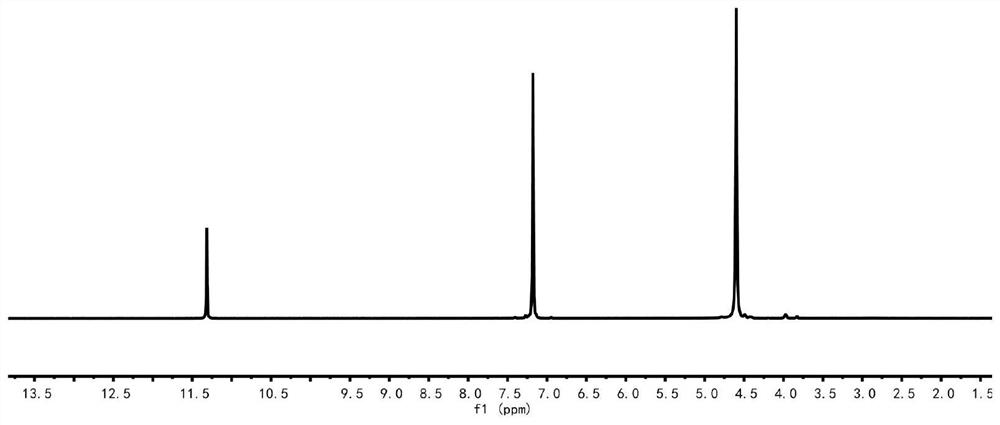
[0081] figure 2 Among them, 11.31ppm is solvent CF 3 The peak of COOD, 7.17ppm is the peak (2H) of hydrogen on the furan ring, and 4.59ppm is the peak (4H) of hydrogen on the ethylene glycol chain segme...
Embodiment 2
[0086] Add dimethyl 2,5-furandicarboxylate, ethylene glycol, pyromellitic anhydride, pentaerythritol and anhydrous cobalt acetate into the reaction kettle in a molar ratio of 1:1.5:0.003:0.001:0.0012, vacuumize, fill Nitrogen was replaced three times, stirring was started and the temperature was gradually raised to 180°C, and the reaction was carried out for 4.5 hours. Then add 0.15% antimony trioxide, 0.2% diphenyl phosphate, and 0.1% antioxidant-168 in the molar weight of dimethyl 2,5-furandicarboxylate, and slowly vacuum to 300Pa~ 2000Pa, pre-polymerized at 225°C for 0.5h. Then gradually raise the temperature to 240° C., and continue the reaction at a vacuum degree below 200 Pa for 2.5 hours to obtain a copolyester containing a furan ring.
[0087] The furan ring-containing copolyester obtained in this embodiment is very light yellow. After testing, its intrinsic viscosity is 0.98dL / g, the tensile strength is 76.8MPa, the tensile modulus is 2.5GPa, and the carbon dioxide g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com