A kind of baicalein activity probe and its synthesis method and application
A synthetic method and technology of baicalein, applied in the field of chemical biology, can solve the problems of unclear and indistinguishable anti-cancer mechanism of baicalein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]1. Baicalein’s "precise anti-cancer" cell and animal model experiments:
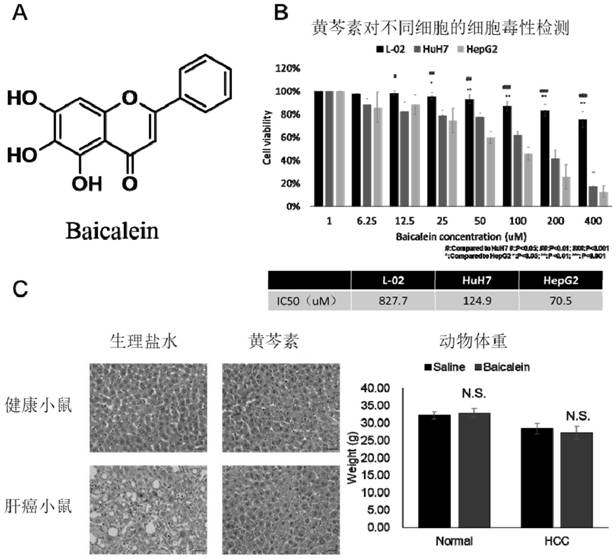
[0035]The applicant has performed proliferation inhibition experiments of baicalein on the immortalized normal liver cell line L-02, liver cancer cell lines HuH7 and HepG2, and calculated the IC50 value. It turns out that asfigure 1 As shown, the cytotoxicity of baicalein to the normal liver cell line L-02 is significantly less than that of the liver cancer cell lines HuH7 and HepG2. In addition, the applicant established a diethylnitrosamine-induced mouse liver cancer model, and then intervened with baicalein, and found that baicalein has a significant therapeutic effect on liver cancer mice, and it has a significant therapeutic effect on normal mice given the same dose. It did not show liver toxicity and did not cause weight loss in mice. Therefore, it can be considered that baicalein is safe to the liver and the whole of mice while showing obvious anti-cancer activity.
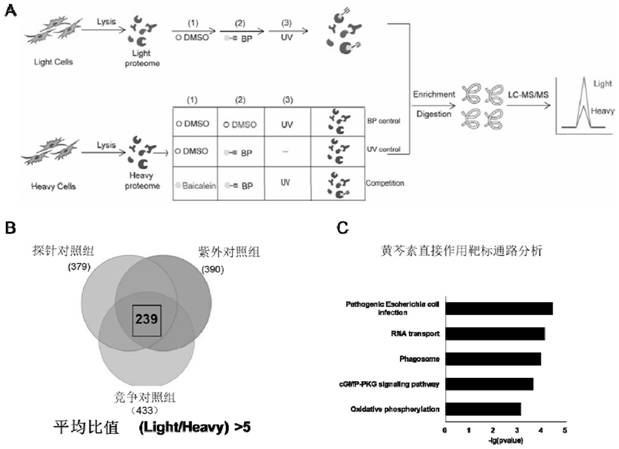
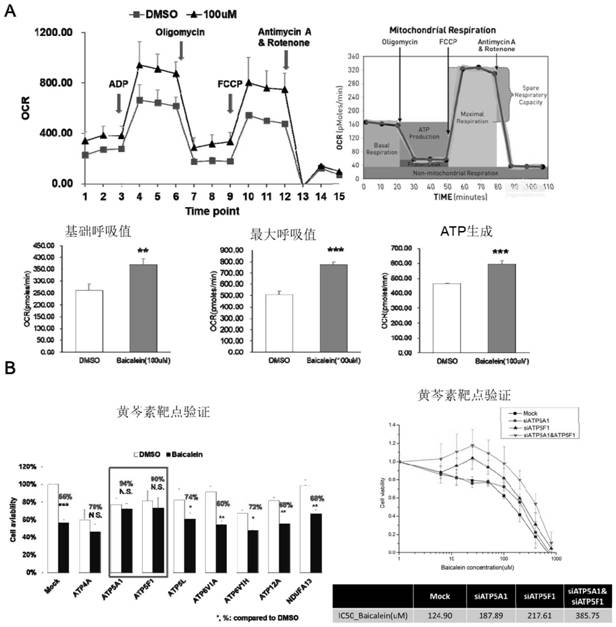
[0036]2. Synthesis and bioequivalenc...
Embodiment 2
[0046]1. Cell culture:
[0047]Hela, HepG2, HuH7 cells (from the Chinese Type Culture Collection (Wuhan, China)) 37°C, 5% CO2Cultured in Dulbecco's modified Eagle medium (DMEM, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin-streptomycin (Thermo Fisher Scientific).
[0048]2. RNA interference
[0049]The siRNA constructs listed below were designed and synthesized by GenePharma (Shanghai, China). In the modeling stage before baicalein treatment, based onRNAiMAX (Thermo Fisher Scientific) protocol for interference.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com