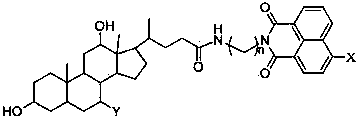
Preparation method of cholic acid-naphthalimide compound
A technology of naphthalimide and compound, which is applied in the field of novel cholic acid-naphthalimide anti-tumor compound and its preparation, can solve the problems of destroying the human immune system, gastrointestinal dysfunction, high recurrence rate, etc., and achieves the preparation method Economical, reduced toxicity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1), preparation of compound 2
[0035] Dissolve 50 mmol of the compound propylenediamine or ethylenediamine in methanol solution with a mass fraction of 10% triethylamine, stir vigorously under ice bath, and add 20 mmol of di-tert-butyl dicarbonate solution in methanol dropwise , the dropwise addition was completed in half an hour. The reaction was naturally warmed to room temperature and stirred overnight. The reaction solution was milky white, and the solvent was evaporated under reduced pressure, and the residue was dissolved in chloroform and washed with saturated Na 2 CO 3 Wash with aqueous solution, dry over anhydrous sodium sulfate, filter, and distill off the solvent under reduced pressure to obtain a light yellow viscous oily liquid, which is mono-Boc-protected propylenediamine or ethylenediamine.
[0036] (2), preparation of compound 3
[0037] Dissolve 1.98g (10mmol) of 1,8-naphthalene dicarboxylic anhydride in 30mL of absolute ethanol, then add 1.74g (...
Embodiment 2
[0046] Embodiment 2: preparation compound 5a
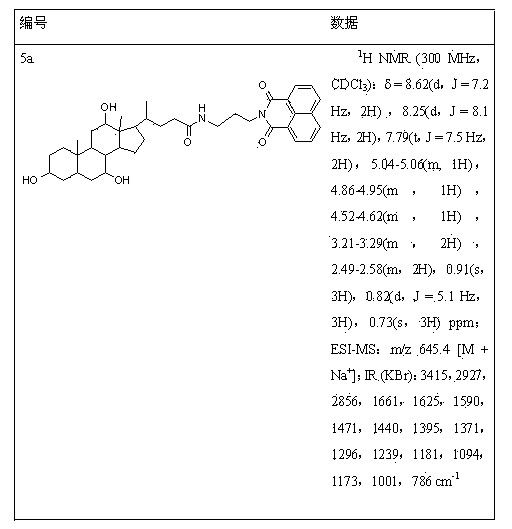
[0047] 0.5 mmol of cholic acid was dissolved in 20 mL of anhydrous dichloromethane, then 0.162 g (1 mmol) of N,N-carbonyldiimidazole (CDI) was added and stirred. Then add 0.5 mmol of compound 4a, drop in 1 mmol of triethylamine, and react at room temperature. TLC detects the progress of the reaction. After the reaction was completed, 30 mL of dichloromethane was added to the reaction solution again, washed with saturated brine, the organic layer was dried over anhydrous sodium sulfate, filtered, and separated by column chromatography (chloroform:ethanol volume ratio was 8:1) to obtain light yellow The solid is compound 5a.
Embodiment 3
[0048] Embodiment 3: preparation compound 5b
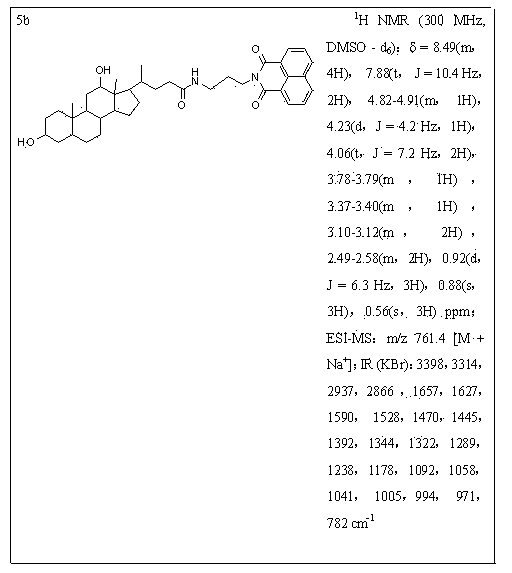
[0049] 0.5 mmol of deoxycholic acid was dissolved in 20 mL of anhydrous dichloromethane, then 0.162 g (1 mmol) of N,N-carbonyldiimidazole (CDI) was added and stirred for one hour. Then add 0.5 mmol of compound 4a, drop in 1 mmol of triethylamine, and react at room temperature. TLC detects the progress of the reaction. After the reaction was completed, 30 mL of dichloromethane was added to the reaction solution again, washed with saturated brine, the organic layer was dried over anhydrous sodium sulfate, filtered, and separated by column chromatography (chloroform:ethanol volume ratio was 6:1) to obtain light yellow The solid is compound 5b.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com