A kind of enzyme-responsive self-assembly peptide and its application
A composition and hydrophobic technology, applied in the field of nanomaterials, can solve the problems of high toxicity and side effects, damage to normal organisms, poor targeting, etc., and achieve the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
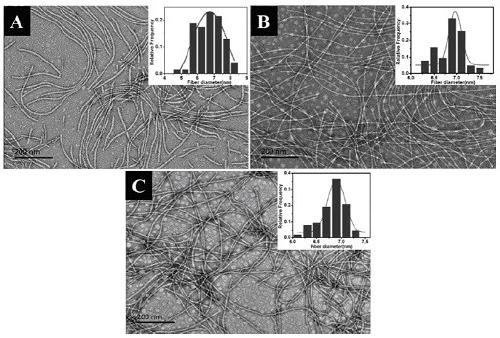
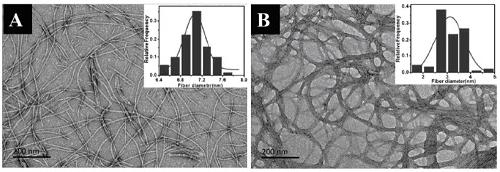
[0027] Embodiment 1: the preparation of polypeptide and nanofiber
[0028] The molecular structure of the polypeptide is as follows
[0029]
[0030] Its molecular synthesis method is as follows:
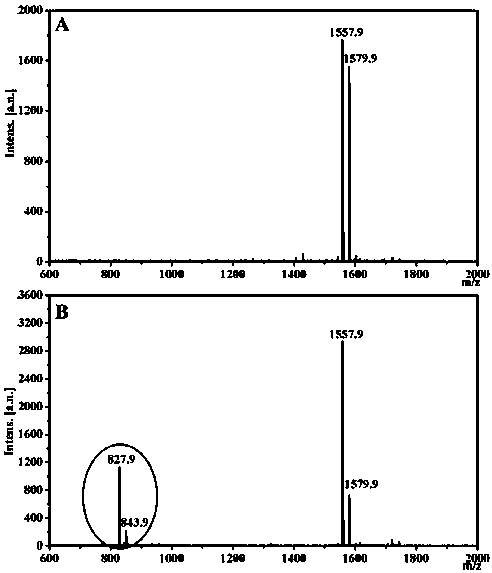
[0031] (1) Weigh 0.7861 g of MBHA resin; prepare a DMF solution of the following substances with a concentration of 0.2 mol / L: A) Fmoc-Phe-OH volume 21 mL, mass 1.63 g; B) Fmoc-Lys(Boc)-OH volume 21 mL, mass 1.97g; C) Fmoc-Pro-OH volume 11 mL, mass 0.74g; D) Fmoc-Leu-OH volume 21 mL, mass 1.48g; E) Fmoc-Gly-OH volume 11 mL, mass 0.65 g; F) Fmoc-Ala-OH volume 11mL, mass 0.68g; G) Fmoc-Arg(Pbf)-OH volume 15mL, mass 1.95g; H) Nap-CH 2 COOH has a volume of 11mL and a mass of 0.41g. Thoroughly stir to dissolve, amino acids are calculated as 2 times the amount to ensure full reaction.
[0032] (2) Prepare the following synthetic reagents: ① Activator: 0.45 mol / L, take 8.54 g of HBTU and 3.04 g of HOBt and dissolve in 50 mL of DMF; ② Activator: 2 mol / L, take 8.7 mL of diisopropyl E...
Embodiment 2
[0037] Embodiment 2: the preparation of pharmaceutical composition
[0038] The method involves mixing a drug molecule solution with Nap-Phe-Phe-Gly-Pro-Leu-Gly-Leu-Ala-Arg-Lys-Arg-Lys-NH 2 Polypeptide Molecule Contact. Solubilization loading of drug molecules by self-assembled structures:
[0039] (1) Dissolve 1 mg of doxorubicin hydrochloride or paclitaxel in 1 mL of dimethyl sulfoxide, mix with 30 μL of triethylamine, and place in the dark at room temperature for 8 hours to obtain a solution.
[0040] (2) Take 5 mg Nap-Phe-Phe-Gly-Pro-Leu-Gly-Leu-Ala-Arg-Lys-Arg-Lys-NH 2 Peptides were added to 500 μL of the above-prepared doxorubicin hydrochloride dimethyl sulfoxide solution or paclitaxel dimethyl sulfoxide solution, and phosphate buffer solution (pH 7.0, ionic strength 10 mM) was used with a dialysis bag (molecular weight cut-off 1 KDa). During dialysis, the buffer solution was changed every 3 hours to remove the unembedded doxorubicin or paclitaxel, and the nano drug c...
Embodiment 3
[0042] Embodiment 3: cell experiment
[0043] (1) Cell experiment: first inoculate 100 uL in a sterile 96-well plate with a density of 1×10 5 Hela (cervical cancer cells), A549 (non-small cell lung cancer cells), NIH 3T3 (mouse embryonic fibroblasts) and COS7 (monkey kidney fibroblasts) cells / mL were placed in a 37°C incubator for 24 hours, After it adheres to the wall, suck out the culture solution in the well plate, add 100uL of fresh culture solution and different volumes of polypeptide nanocarrier solution embedded with doxorubicin into each well, and set up 4 parallel wells for each concentration. Abs drug , In addition, the wells that only added buffer Tris and no peptide were used as the control group Abs Tris , and then put the orifice plate back in the incubator at 37°C for 24 hours. After the effect was completed, add 20 uL of MTT solution with a concentration of 5 mg / mL to each well, and continue to cultivate in the incubator for 4 hours. After that, the liqui...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com