Method for purifying dihydroxynaphthalene
A technology of dihydroxynaphthalene and purification method, applied in chemical instruments and methods, preparation of organic compounds, gas treatment, etc., can solve problems such as poor economy, decreased purification ability of composition, filter blockage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
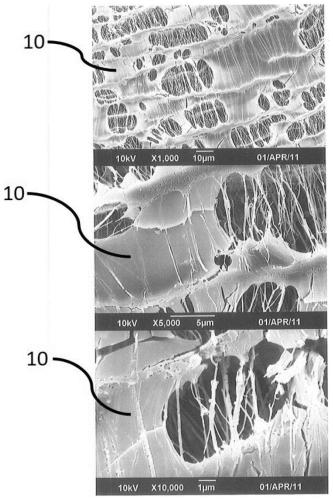
Image
Examples
Embodiment 1-1
[0061] 72.2g of commercially available 1,5-dihydroxynaphthalene (hereinafter referred to as 15DHN, sulfur element content 400ppm, measured by ICP-OES) was dissolved in 127.6g of propylene glycol methyl ether (hereinafter referred to as PGME) and 353.6g A mixed solvent of methyl isobutyl ketone (hereinafter referred to as MIBK). 57.2 g of neutral alumina (Tomita AD-250NS manufactured by Tomita Pharmaceutical co., Ltd., particle size 60-250 μm, pH 7.5) was added thereto, stirred at room temperature for 12 hours, and the neutral alumina was collected by filtration. , The obtained 15DHN PGME / MIBK solution was washed with ion-exchanged water, PGME was added, and concentrated to obtain 500 g of 13 wt% 15DHN-PGME solution (purification raw material 1). The sulfur element content per part of the solid content of the 15DHN solution was measured by ICP-OES, and the result was 45 ppm.
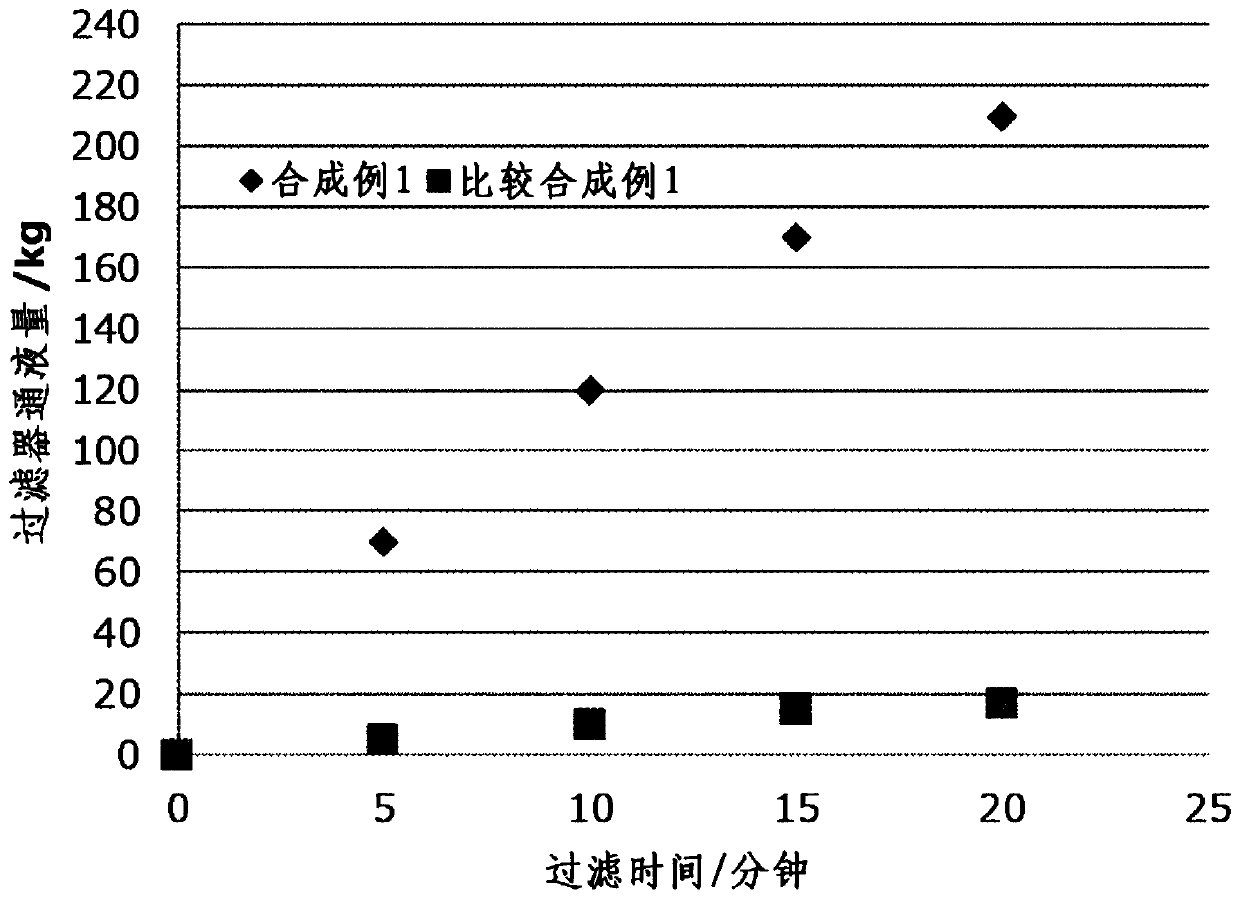
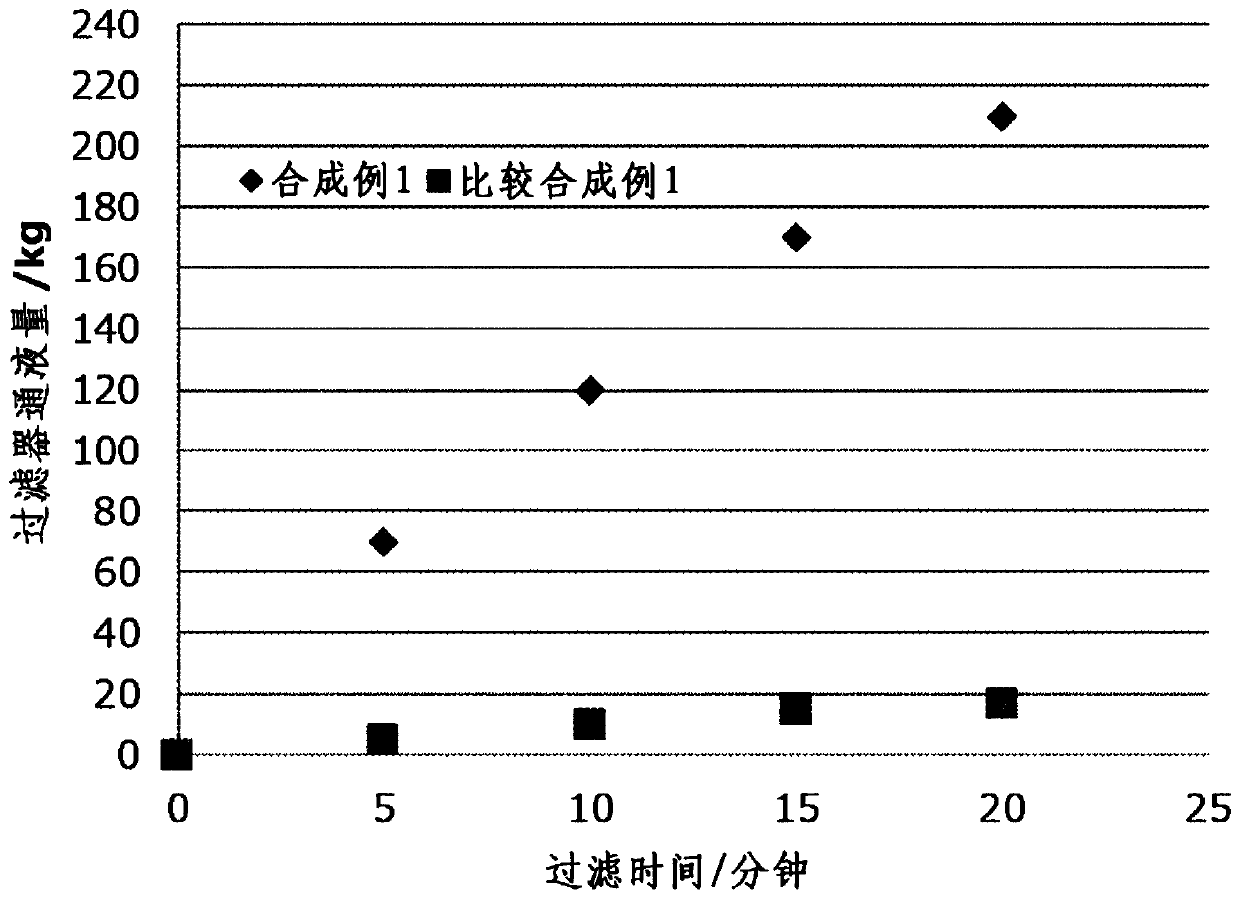
Synthetic example 1
[0094]In a 1000ml flask, mix the 13wt% 15DHN-PGME solution (purification raw material 1), 2.8g of p-toluenesulfonic acid and the PGME of 2.8g purified in the embodiment 1-1 of 500g (0.5 mole), at 80 ℃ Next, 14.3 g of 50 wt % formaldehyde aqueous solution was added with stirring. After maintaining the temperature at 80° C. and stirring for 6 hours, it was cooled to room temperature (monomer conversion rate 77%). After the obtained solution was concentrated under reduced pressure, 540 g of hexane was added to separate and precipitate the polymer component. After filtering and removing the upper filtrate, this operation was repeated to make the residual monomers less than 5%. The remaining polymer component was redissolved in 200 g of MIBK, and 200 g of ion-exchanged water was added to remove the metal ion component. 300 g of propylene glycol monomethyl ether acetate (PGMEA) was added to the MIBK solution from which metal ion components were removed, and concentrated under redu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com