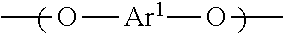Patents
Literature
310 results about "Hydroxynaphthalenes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
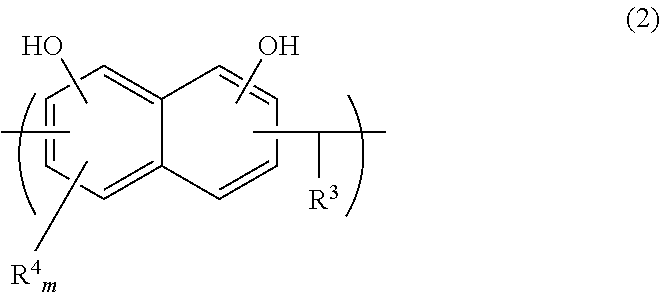
Resins and photoresist compositions comprising same
InactiveUS7244542B2Improve photolithographic effectConvenient and smoothPhotosensitive materialsRadiation applicationsArylResist
Provided are new resins that comprise carbocyclic aryl units with hetero substitution units and photoresists that contain such resins. Particularly preferred photoresists of the invention comprise a deblocking resin that contains hydroxy naphthyl units and can be effectively imaged with sub-200 nm radiation such as 193 nm radiation.
Owner:SHIPLEY CO LLC
Liquid crystalline polyester resin composition
InactiveUS20060025561A1Improve moldabilityImprove productivityLiquid crystal compositionsMicaLiquid crystalline
The object is to provide a liquid crystalline polymer material having good moldability, excellent flatness, warp-deformation resistance, and heat-resistance, and securing performance balance. A specified quantity of mica and a fibrous filler is added to a liquid crystalline polyester resin prepared by combining a 2-hydroxy-6-naphthoic acid unit and a 4-hydroxybenzoic acid unit at a specifically limited ratio.
Owner:POLYPLASTICS CO LTD
Positive resist composition and patterning process
ActiveUS20120220112A1Improve adhesionExpand coveragePhotosensitive materialsSemiconductor/solid-state device manufacturingResistImage resolution
A positive resist composition based on a polymer comprising recurring units of (meth)acrylate having a cyclic acid labile group and a dihydroxynaphthalene novolak resin, and containing a photoacid generator is improved in resolution, step coverage and adhesion on a highly reflective stepped substrate, has high resolution, and forms a pattern of good profile and minimal edge roughness through exposure and development.
Owner:SHIN ETSU CHEM IND CO LTD
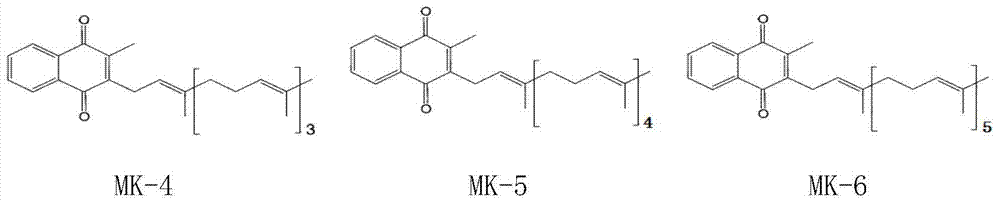
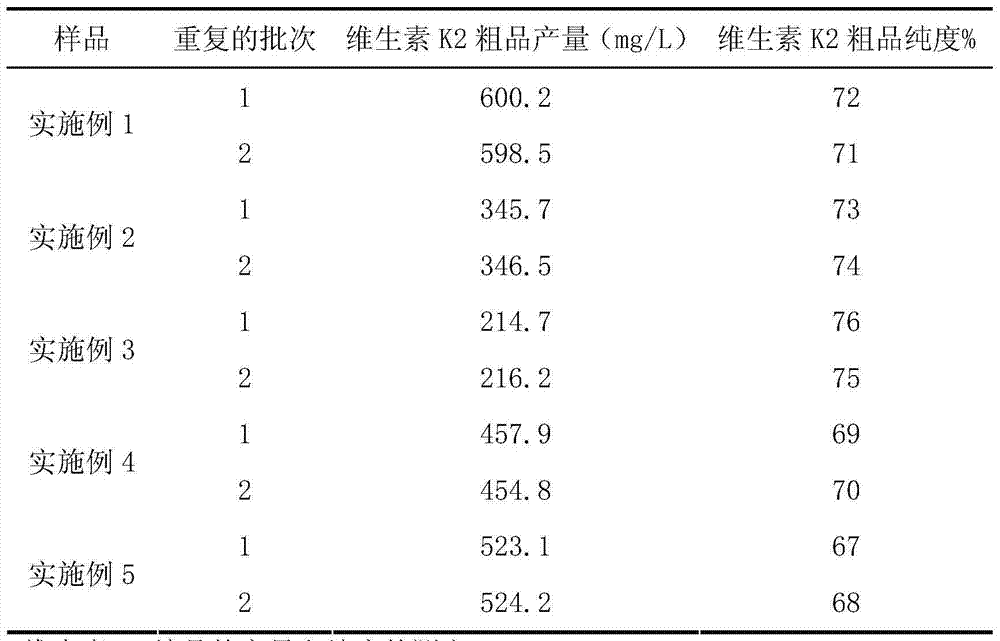
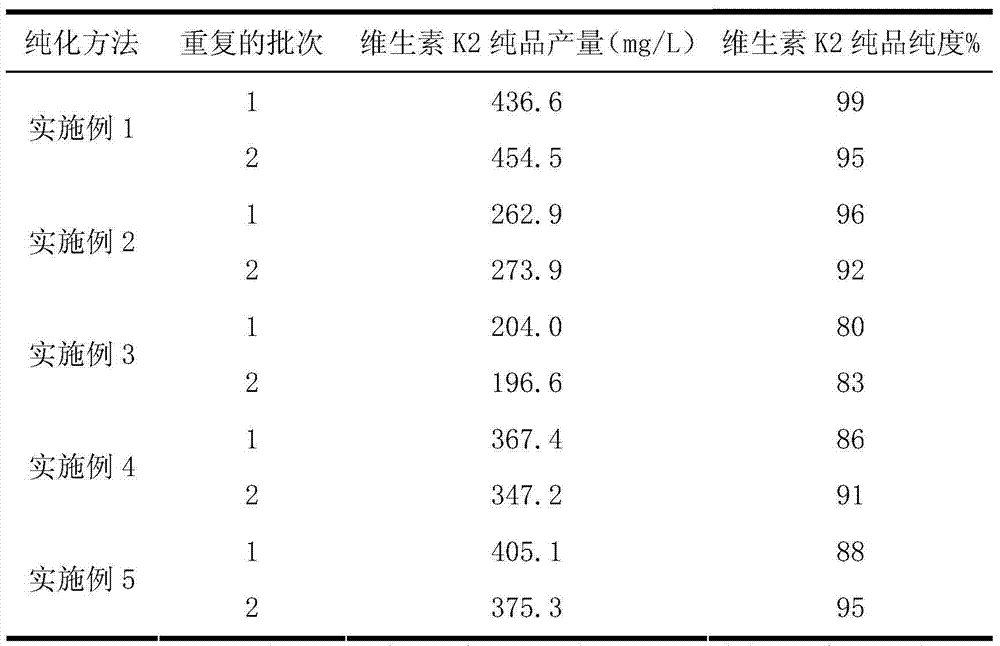
Vitamin K2 and preparation process thereof
ActiveCN103571897AHigh purityIncrease productionMicroorganism based processesFermentationVitamin K2Organic solvent
The invention discloses a vitamin K2 and preparation process thereof. According to the process, 1-hydroxyl-2-naphthoic acid resistant flavobacterium strain HNA12-D is fermented and cultured, and vitamin K2 efficiently metabolizes in the strain through the batch feeding of glycerin serving as the carbon source, peptone serving as the nitrogen source and precursor substances, and is extracted by using organic solvents and separated by macroporous resin, and the yield and purity of the product are improved. The total output of the vitamin K2 crude product is 200-600 mg / L after the flavobacterium strain HNA12-D of the invention is fermented and cultured for 120-144 hours, and the product purity reaches 80-99 percent after absorption chromatography; compared with the prior art, the yield output and the product purity are greatly improved, and the product can be used in industrial production.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
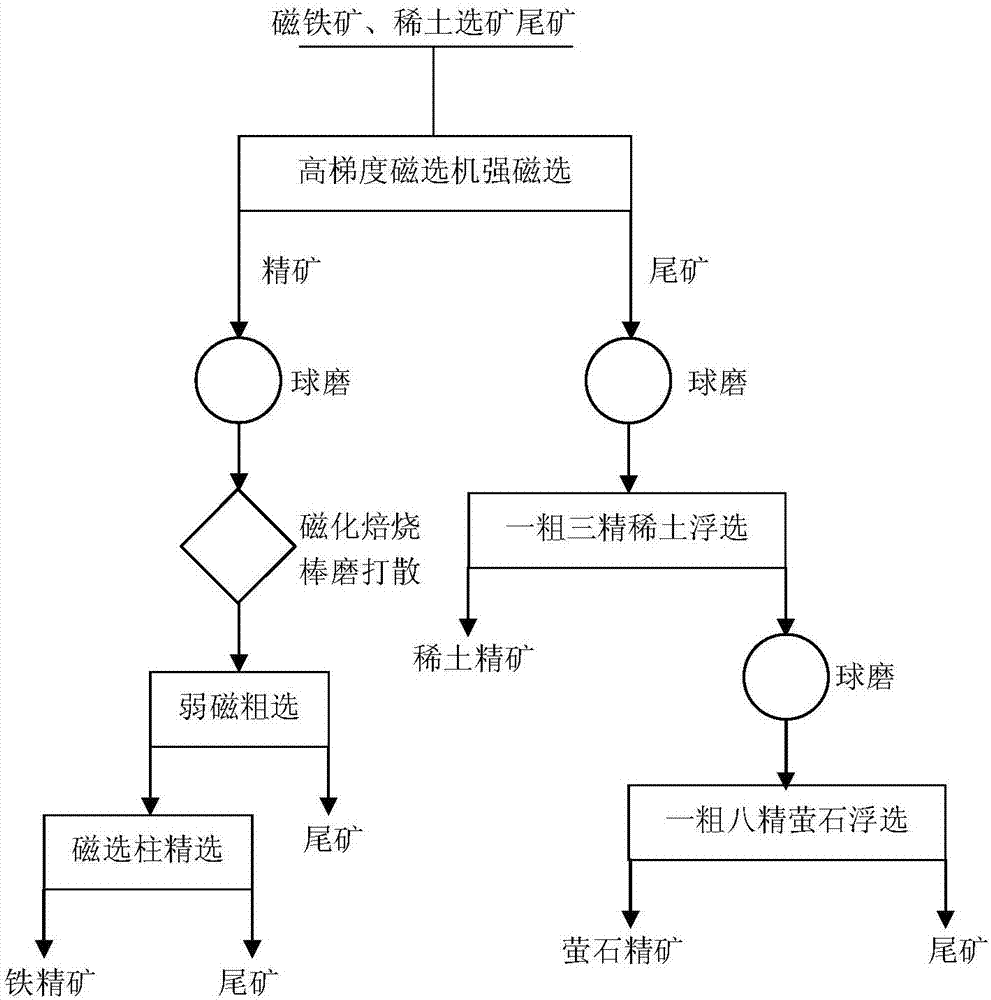
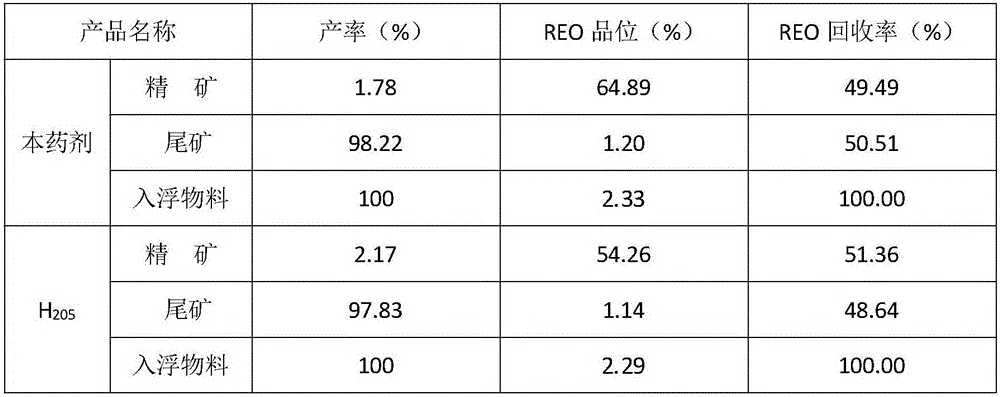
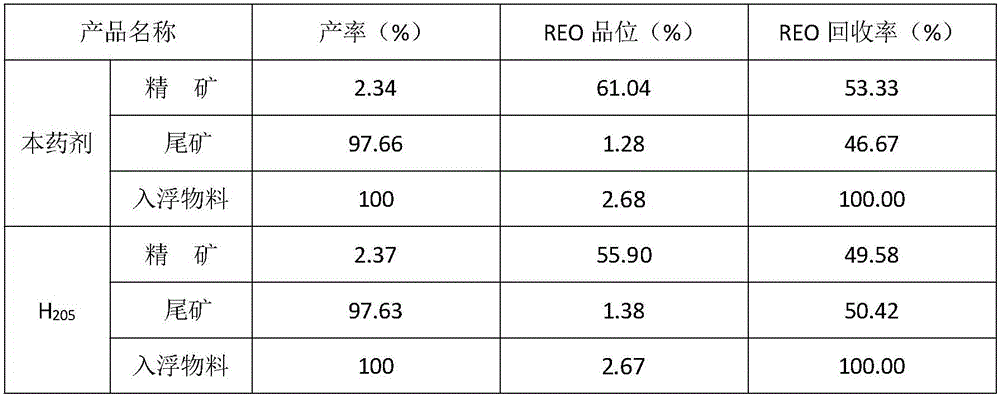
Mineral processing method for comprehensively recovering weak magnetic iron, rare earth and fluorite
InactiveCN107282288ARealize comprehensive utilizationHigh gradient magnetic separationFlotationMagnetic separatorSodium silicate
The invention discloses a mineral processing method for comprehensively recovering weak magnetic iron, rare earth and fluorite. The mineral processing method comprises the following steps: by taking tailings as a raw material, adopting high intensity magnetic separation by a high gradient magnetic separator, ore grinding, magnetic roasting, low intensity magnetic separation by a cylinder magnetic separator and selection by a magnetic separation column to obtain final iron concentrates with a TFe grade being 60-62 percent; adopting ore grinding on the tailings subjected to high intensity magnetic separation by taking o-hydroxy naphthalene hydroxamic acid as a collector and water glass as an inhibitor, and carrying out flotation with one rough floatation and three concentrating to obtain rare earth concentrates with an REO grade being 48-52 percent; and adopting ore grinding on rare earth flotation tailings by taking sodium oleate as a collector and acidified sodium silicate as an inhibitor, and carrying out flotation with one rough floatation and eight concentrating to obtain fluorite concentrates with a CaF2 grade being 92-94 percent. By adopting a technology provided by the invention, iron, rare earth and fluorite concentrates which can be directly sold can be obtained, comprehensive utilization of useful resources in the tailings is realized, and the mineral processing method has important economic value and environmental significance.
Owner:INNER MONGOLIA UNIV OF SCI & TECH
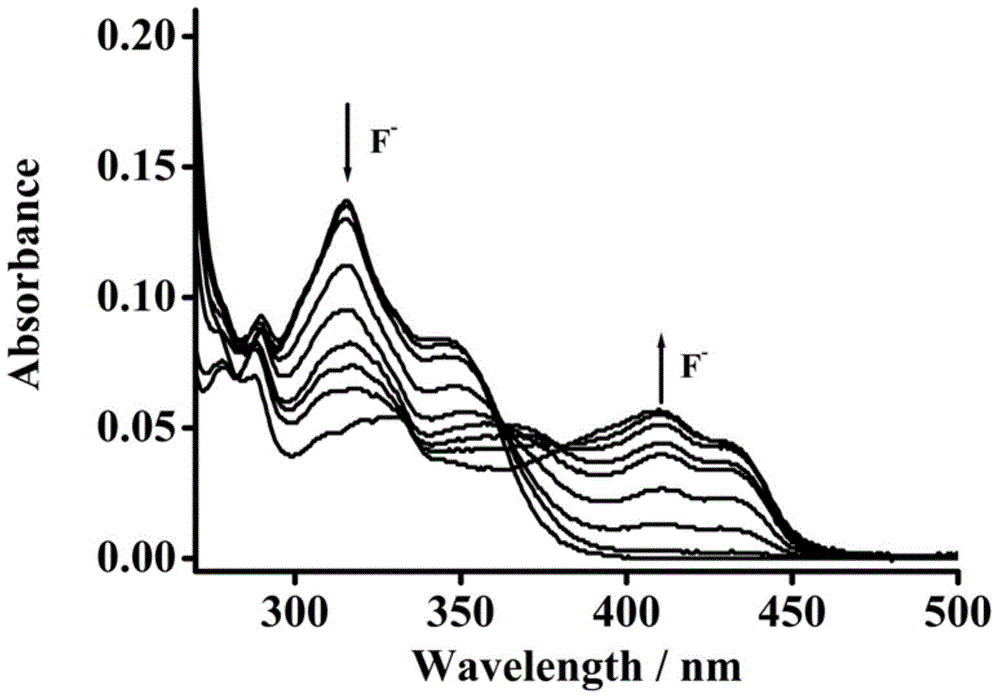
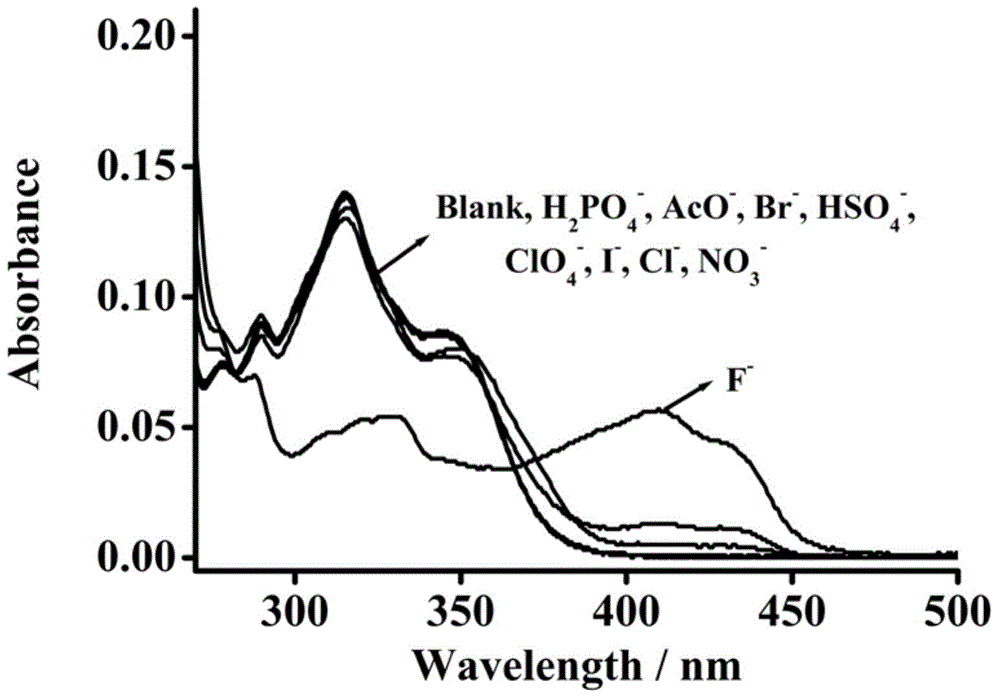
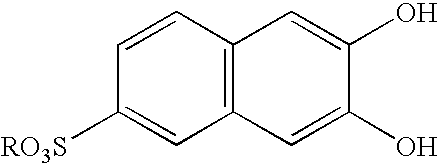
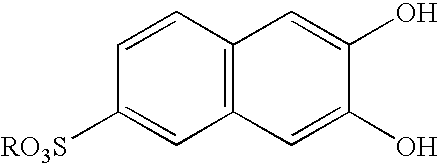
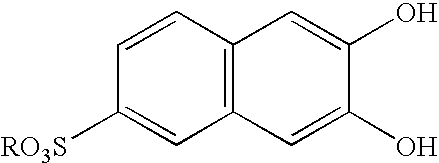
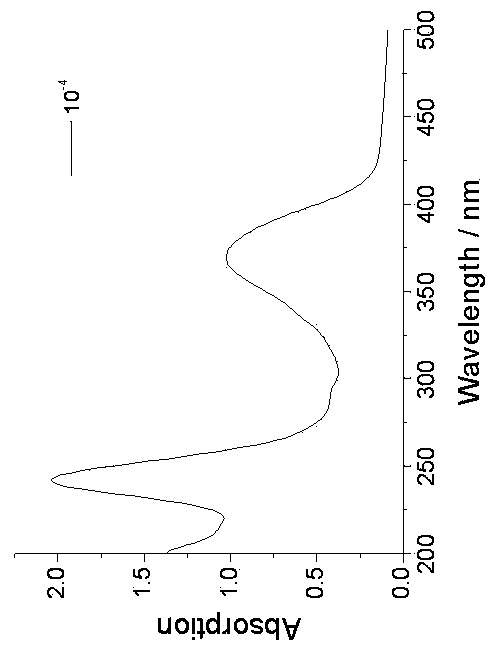
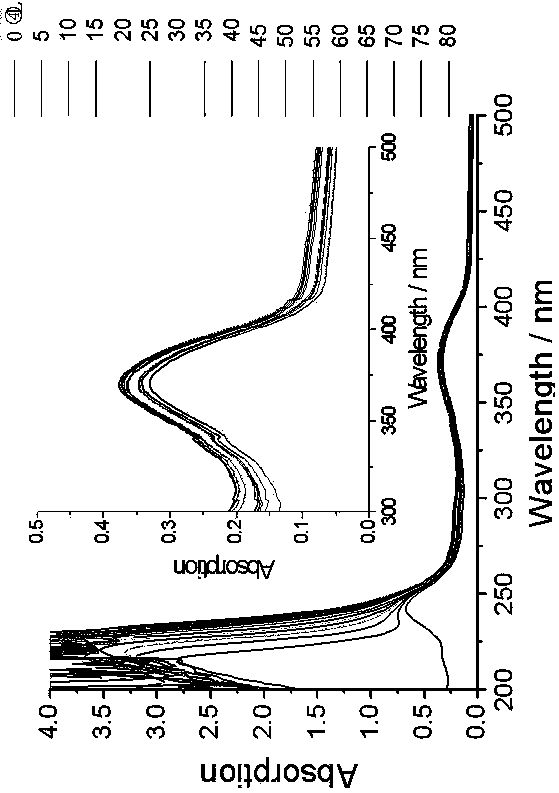
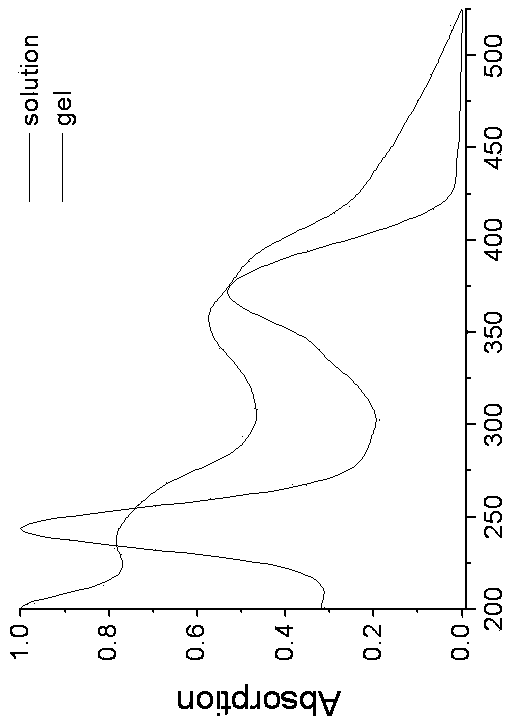
Pillar[5]arene and 2-hydroxy-3-naphthoic acid complex and preparation thereof and application in detecting iron ions and fluorine ions
InactiveCN105753662AOrganic compound preparationCarboxylic compound preparationMolecular fluorescenceFluorescent quenching
The invention provides a pillar[5]arene and 2-hydroxy-3-naphthoic acid complex.Copolymerized pillar[5]arene serves as a subject, 2-hydroxy-3-naphthoic acid serves as an object, C-H......pi and electrostatic attraction serve as driving force, partial 2-hydroxy-3-naphthoic acid provided with negative charges is included into a cavity of pillararene in an alkaline environment, the assembling process of the subject and the object is completed, and the stable pillar[5]arene and 2-hydroxy-3-naphthoic acid complex is formed.Iron ions are added to an alkaline aqueous solution of the complex, and the subject and the object are subjected to fluorescence quenching; fluorine ions are added, a complex is formed by the fluorine ions and the iron ions, so that the iron ions are separated from the cavity of copolymerized pillar[5]arene, and molecular fluorescence of the complex is reopened.Therefore, Fe<3+> can be determined through the pillar[5]arene and 2-hydroxy-3-naphthoic acid complex, and F<-> can be continuously identified through fluorescence quenching of the solution.
Owner:NORTHWEST NORMAL UNIVERSITY
Controlled release composition and method of producing the same
InactiveUS20030134800A1Suppressing initial excess releaseStable releasing speed for a long period of timeNervous disorderTetrapeptide ingredientsControlled release3-hydroxy-2-naphthoic acid
A controlled release composition containing a physiologically active substance in high content, suppressing the initial excess release, and achieving a stable release speed over a long period of time is provided. A controlled release composition comprising (1) a physiologically active substance or salt thereof in an amount of about 14% (w / w) to about 24% (w / w) based on the total composition weight, (2) hydroxynaphthoic acid selected from the group consisting of 3-hydroxy-2-naphthoic acid and 1-hydroxy-2-naphthoic acid or salt thereof, and (3) a lactic acid polymer or salt thereof having a weight-average molecular weight of 15000 to 50000 in which the content of polymers having molecular weights of 5000 or less is about 5% by weight or less, wherein the molar ratio of said hydroxynaphthoic acid or salt thereof to said physiologically active substance or salt thereof is from 3:4 to 4:3.
Owner:TAKEDA PHARMA CO LTD
Thermotropic liquid-crystalline polymer
InactiveUS7179401B2Less anisotropy in mechanical strengthImprove welding strengthLiquid crystal compositionsMonocomponent copolyesters artificial filamentLiquid crystallinePolymer science
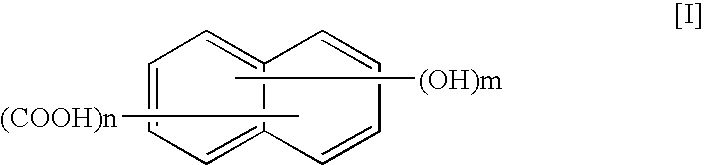
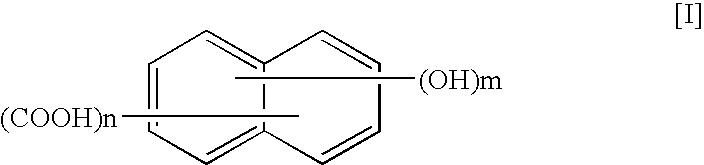
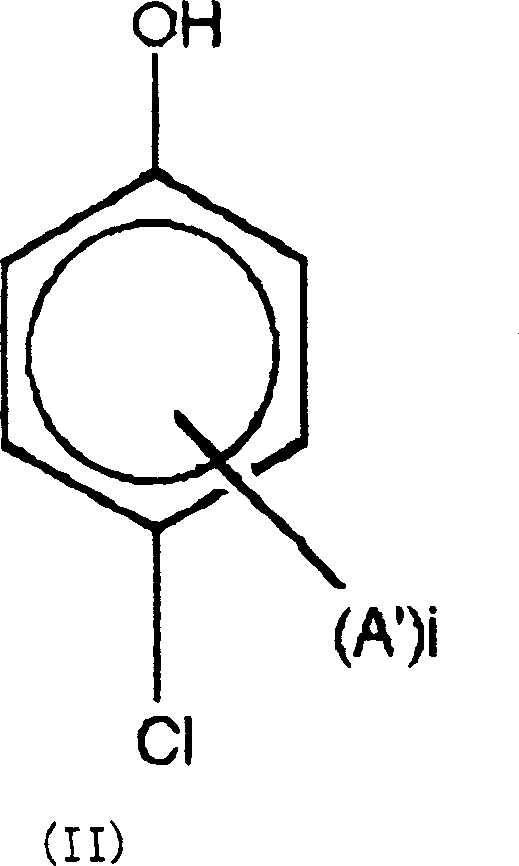
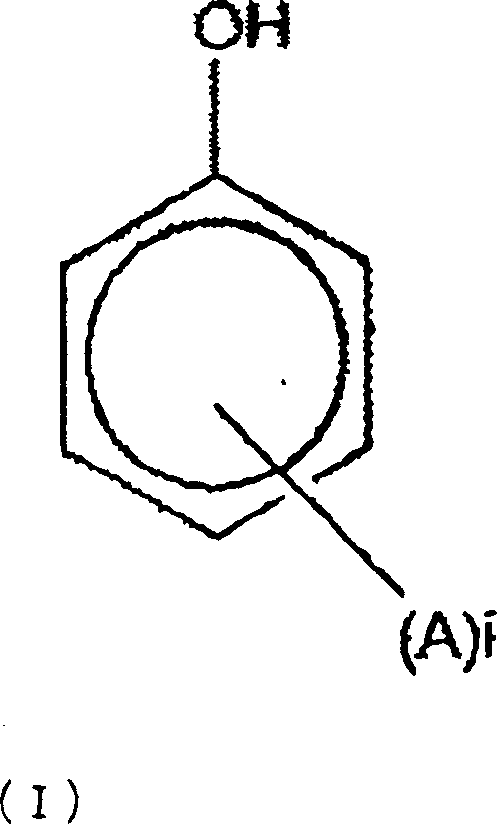
A thermotropic liquid crystalline polymer, which is obtainable by copolymerizing a trifunctional hydroxynaphthalene carboxylic acid represented by the general formula (I) and / or reactive derivative thereof with other polymerizing monomer(s)wherein one of m and n is 1 and the other is 2, and a composition including the polymer are provided. The molded article obtained from the thermotropic liquid crystalline polymer or the composition has less anisotropy in mechanical strength and improved weld strength.
Owner:UENO FINE CHEM IND LTD
1-(3-(6-(3-hydroxynaphthalen-1-yl)benzofuran-2-yl)azetidin-1 yl)prop-2-en-1-one derivatives and similar compounds as kras g12c modulators for treating cancer
ActiveUS20190382377A1Prevent proliferationOrganic chemistryPharmaceutical delivery mechanismDiseaseBenzoxazole
Compounds having activity as inhibitors of G12C mutant KRAS protein are provided. The compounds have the following structure (I): or a pharmaceutically acceptable salt, isotopic form or stereoisomer thereof, wherein A is a five-membered heteroaryl comprising 1 or 2 non-adjacent heteroatoms, inclusive of X and Y; W, X, Y, Z, L, L1, E, R1, R2bR2c and the dotted circle are as defined herein. Methods associated with preparation and use of such compounds, pharmaceutical compositions comprising such compounds and compounds for use in methods to modulate the activity of G12C mutant KRAS protein for treatment of disorders, such as cancer, are also provided. Preferred compounds are e.g. 1-(3-(6-(3-hydroxynaphthalen-1-yl)benzofuran-2-yl)azetidin-1yl)prop-2-en-1-one derivatives and related compounds such as e.g. the corresponding derivatives with e.g. a benzoimidazole, indole, benzooxazole, imidazopyridine or imidazole core structure, substituted on ring A by e.g. azetidine, pyrrolidine, azepane or bicyclopentane-amine (L1) each substituted by e.g. propenone (E), and the core structure substituted on the six-membered ring with e.g. 3-hydroxynaphthalene or indazole or hydroxy-, alkoxy- and / or fluoro-substituted phenyl (R1).
Owner:ARAXES PHARMA LLC
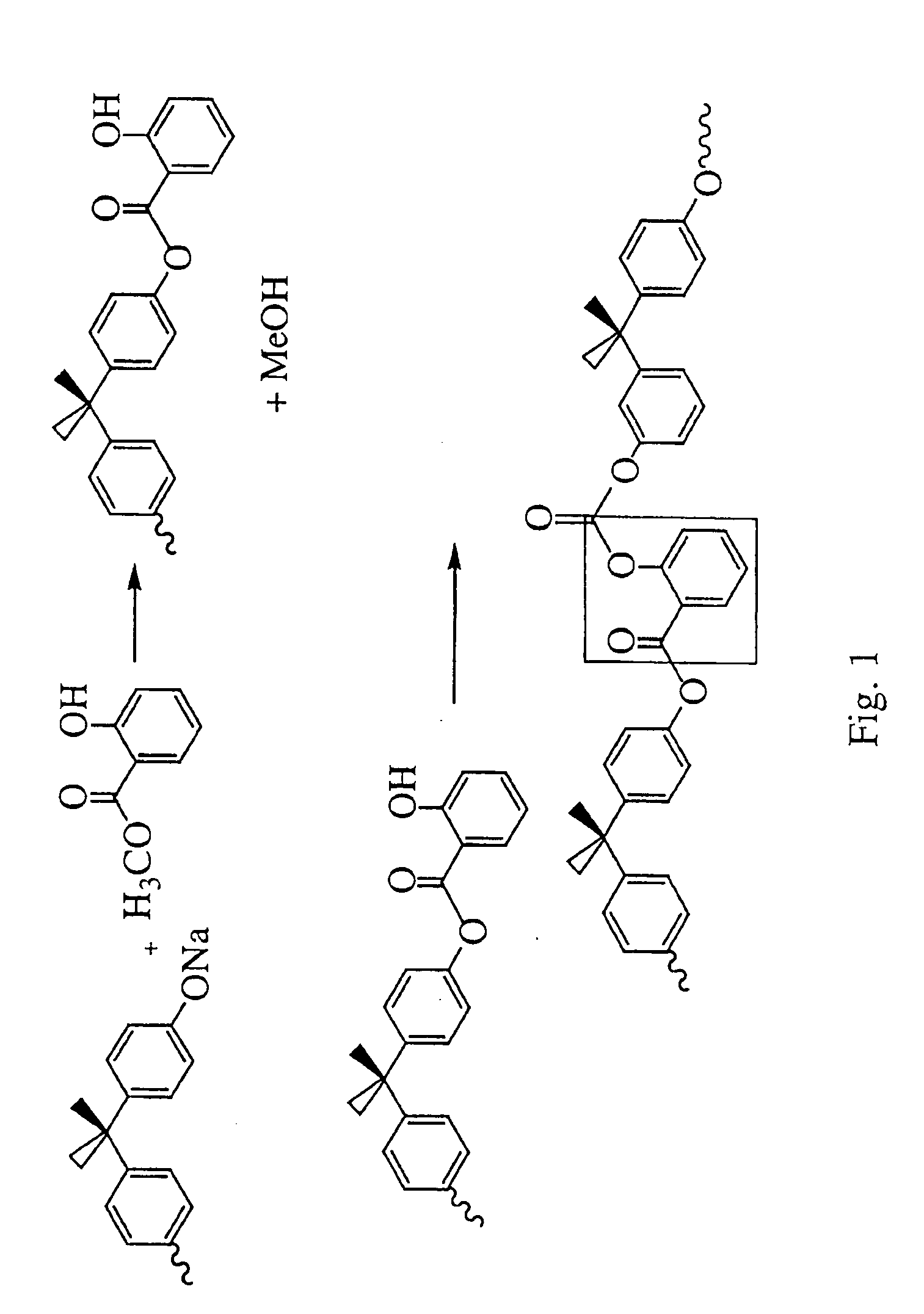
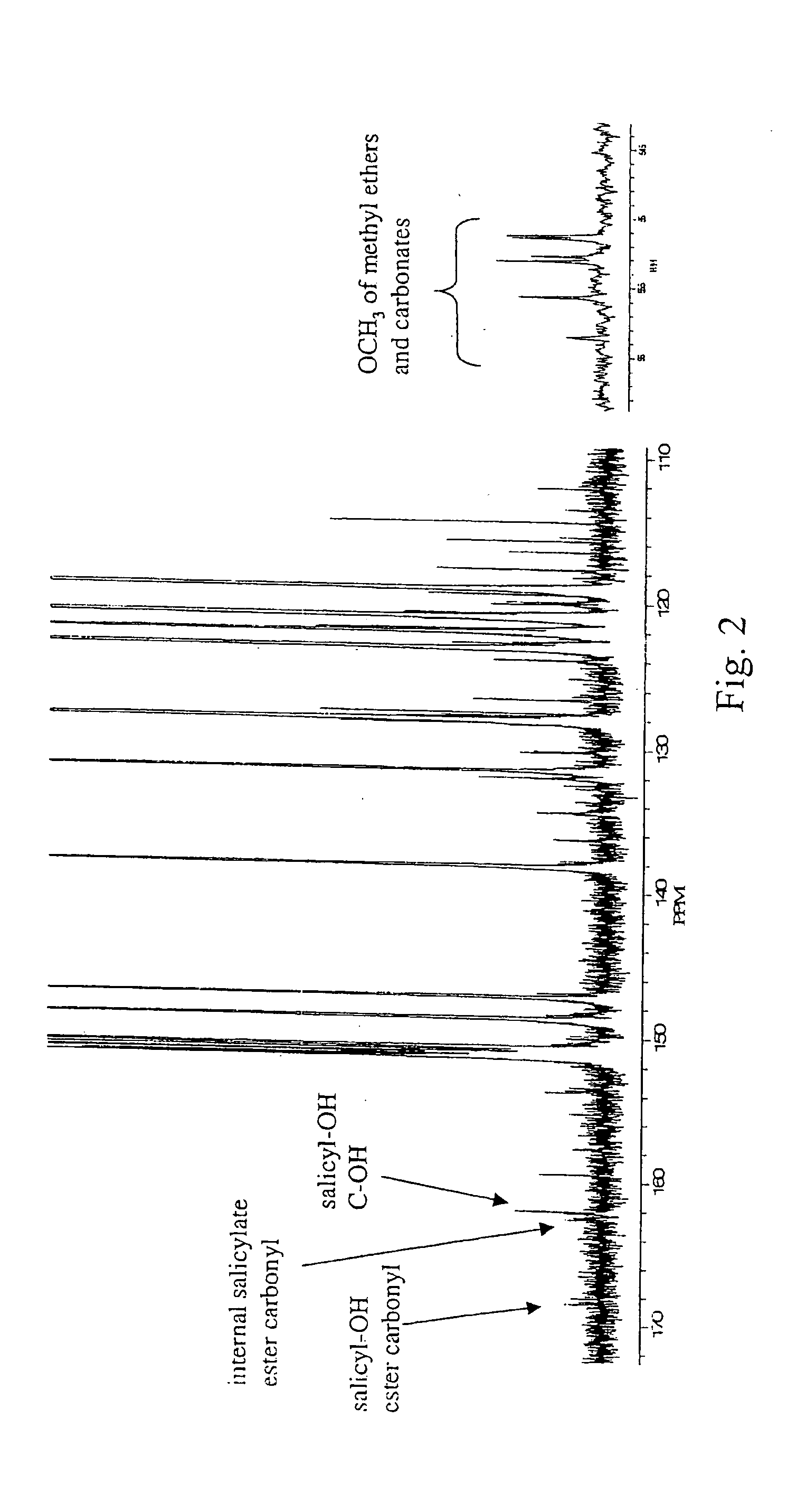
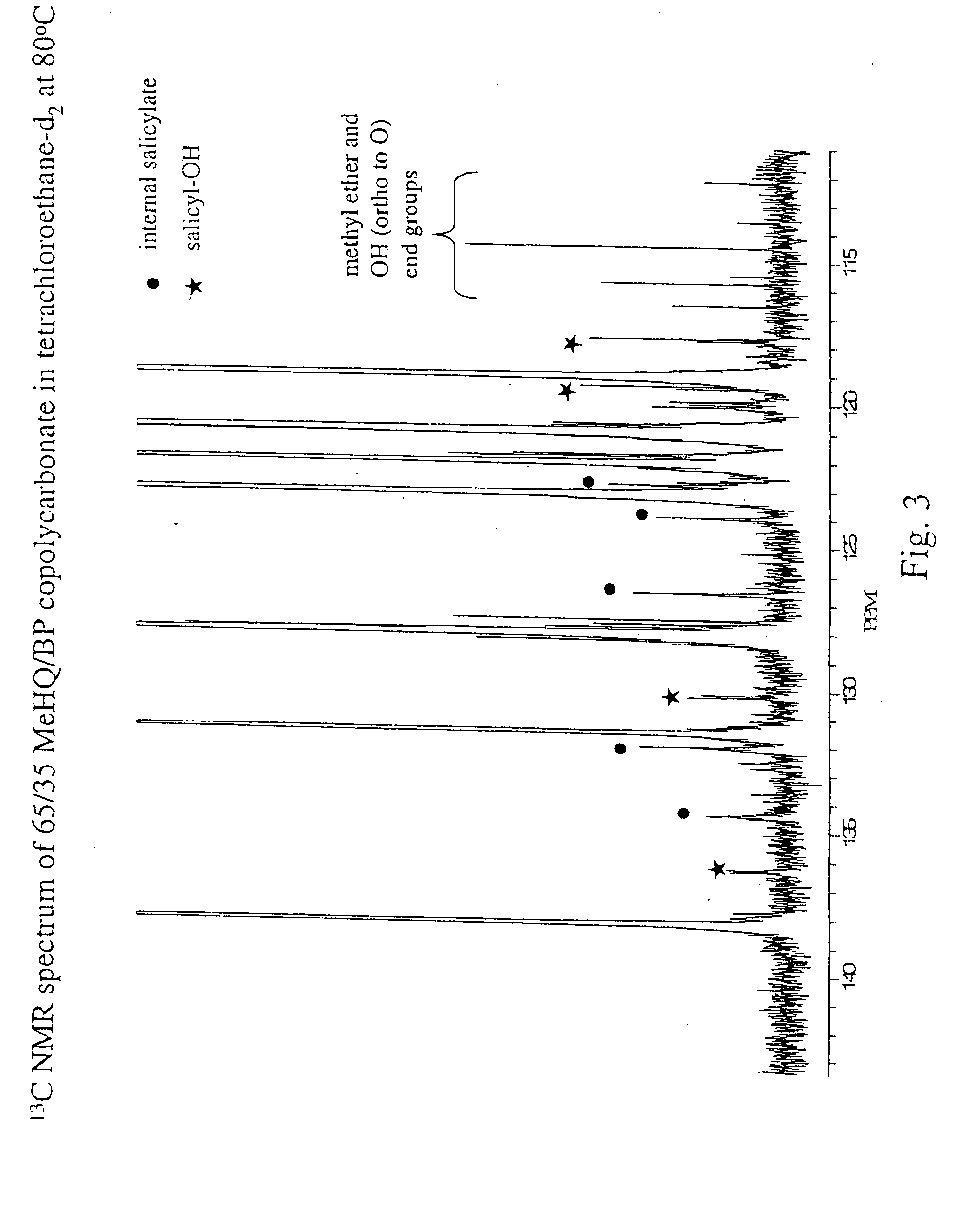
Liquid crystal polycarbonates and methods of preparing same
ActiveUS20050192424A1Liquid crystal compositionsOrganic-compounds/hydrides/coordination-complexes catalystsHydroquinone CompoundDiol
Liquid crystal polycarbonates are made by forming a reaction mixture containing (a) an activated diaryl carbonate; (b) at least two species of aromatic diols selected from among resorcinol, 4,4′-biphenol, hydroquinone, methylhydroquinone, 4,4′-dihydroxyphenylether, dihydroxynaphthalene, including in particular the 2,6, 1,5, and 2,7 isomers, 4,4′-dihydroxybenzophenone and 2,6-dihydroxyanthraquinone (anthraflavic acid); and (c) optionally bisphenol A in a maximum amount of 10 mole %; and processing the reaction mixture in a melt transesterification reaction to form a liquid crystal polycarbonate. While the product composition has the same overall characteristics as compositions made using diphenyl carbonate as the donor moiety for the carbonate linkage, they are analytically distinguishable because of limited incorporation of intermediate or end-cap residues derived from the activated diaryl carbonate.
Owner:SHPP GLOBAL TECH BV +1
Specific fluorescence probe substrates of human carboxylesterase 2 and application thereof
InactiveCN104120164AEasy to detectThe synthesis process is simpleOrganic chemistryMicrobiological testing/measurementMetaboliteHydrolysis
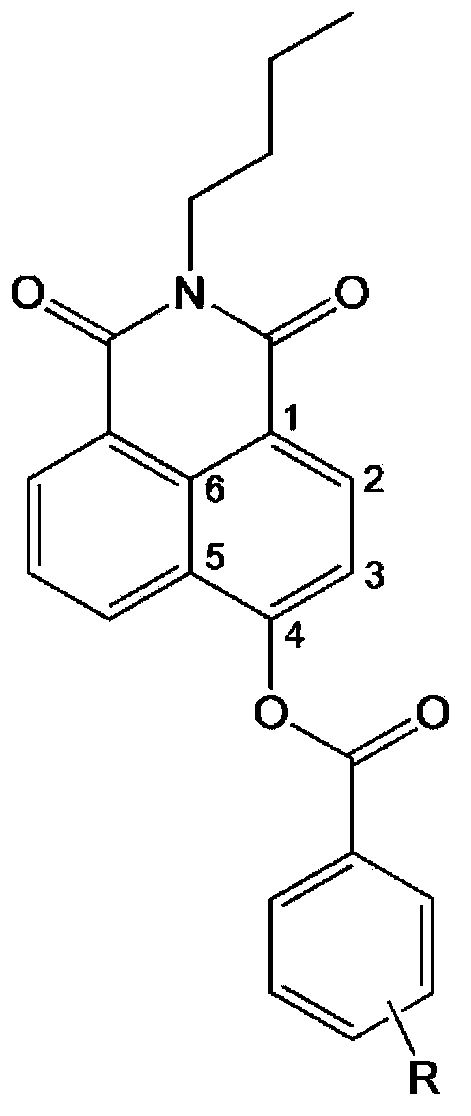
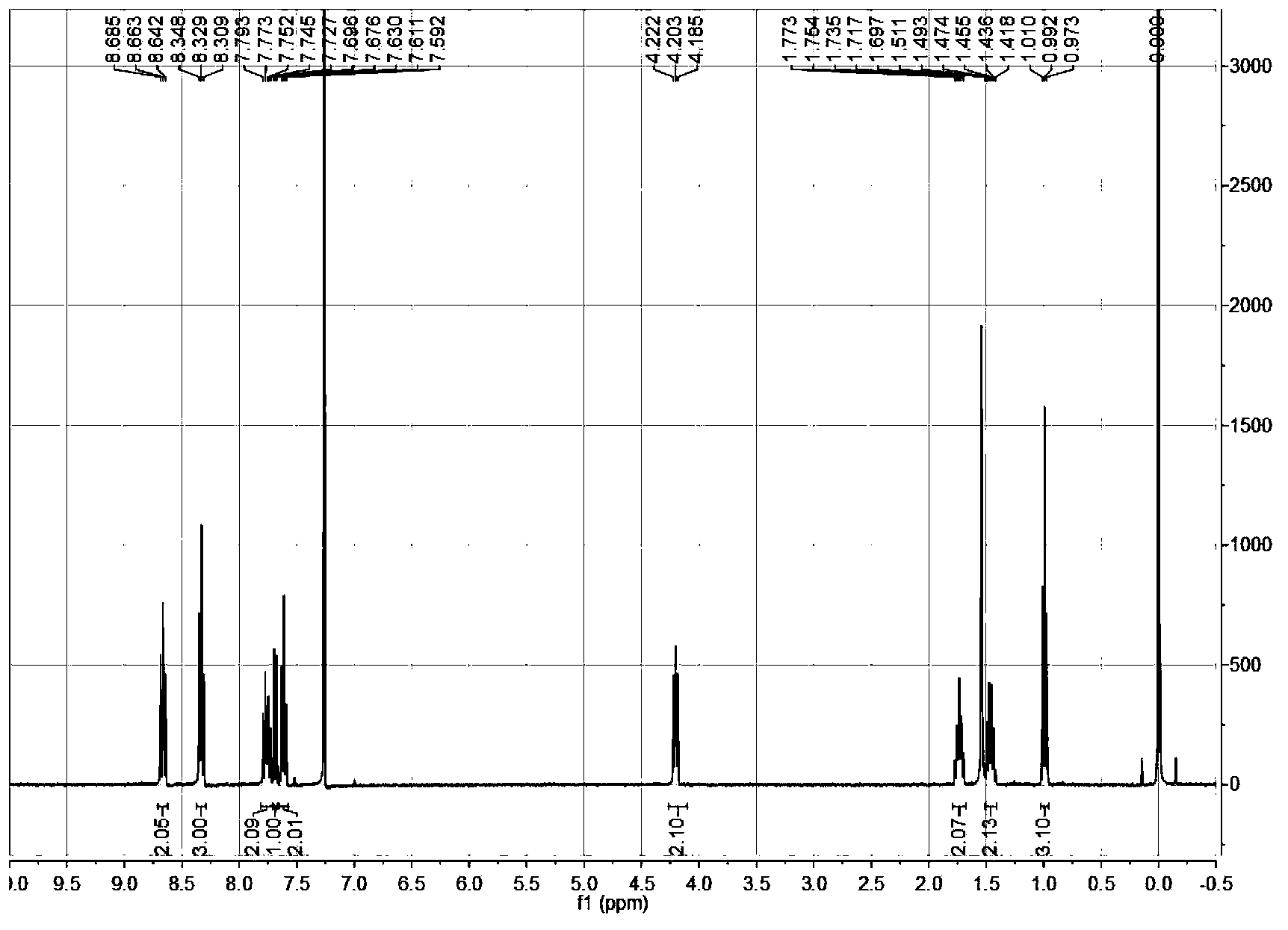
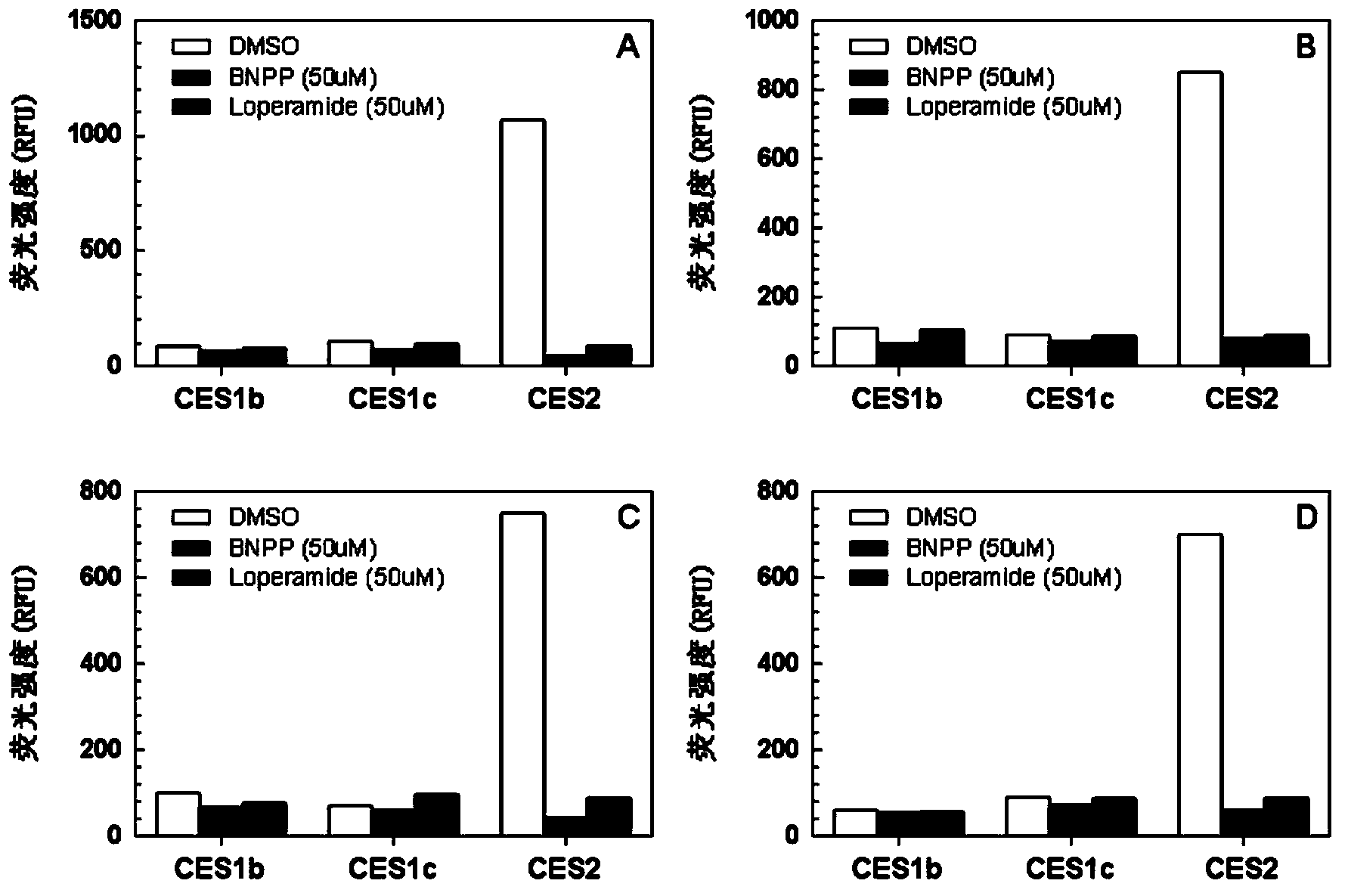
The invention provides a specific fluorescence probe substrates of human carboxylesterase 2 (CES2) and application thereof. The specific probe substrate is a benzoateb compound of a C4 hydroxyl naphthalimide, and is applicable to determine the enzyme activity of CES2 in a biological system. The CES2 enzyme activity determination flow comprises: selecting a hydrolysis benzoyl-removal reaction of the benzoate compound of the C4 hydroxyl naphthalimide as a probe reaction, and quantitatively determining the generation amount of a hydrolysis metabolite of the compound in a unit time, so as to determine the enzyme activity of CES2 in all biological samples, cells, bodies and integral organs. The probe is applicable to quantitative assessment of CES2 enzyme activity in biological samples of different species and different individual sources, and quantitative determination on CES2 enzyme activity in different sources of animal tissue cell culture fluids and cell preparation substances, so that the probe is expected to help to realize assessment on medicine disposal capability of important drug metablic enzyme CES2. Additionally, the probe also is applicable as an inhibitor for rapidly screening CES2 in vitro by means of the probe reaction.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
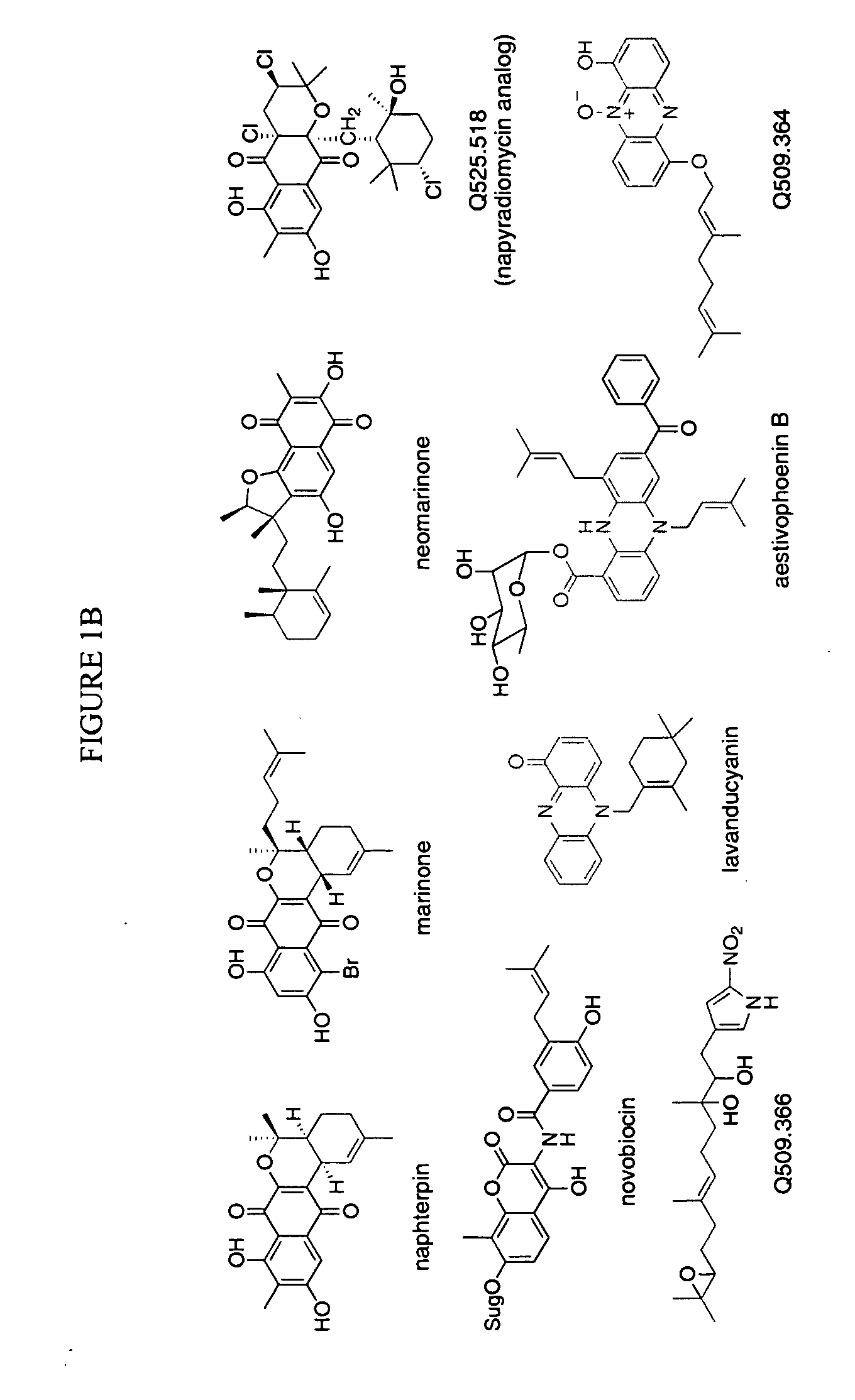
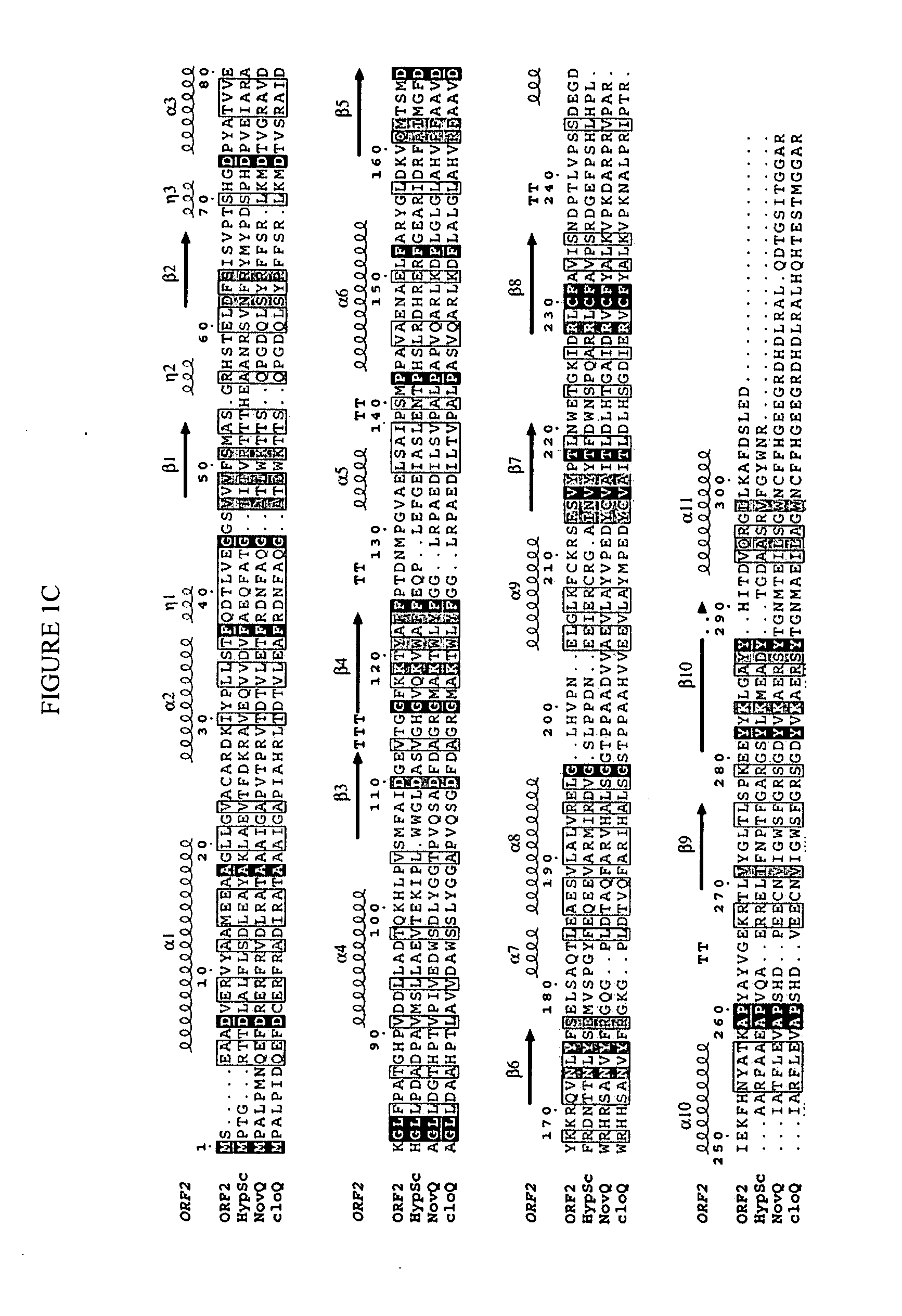
Novel aromatic prenyltransferases, nucleic acids encoding same and uses therefor
ActiveUS20060183211A1Sugar derivativesMicrobiological testing/measurementPrenyltransferase activityIsoprene
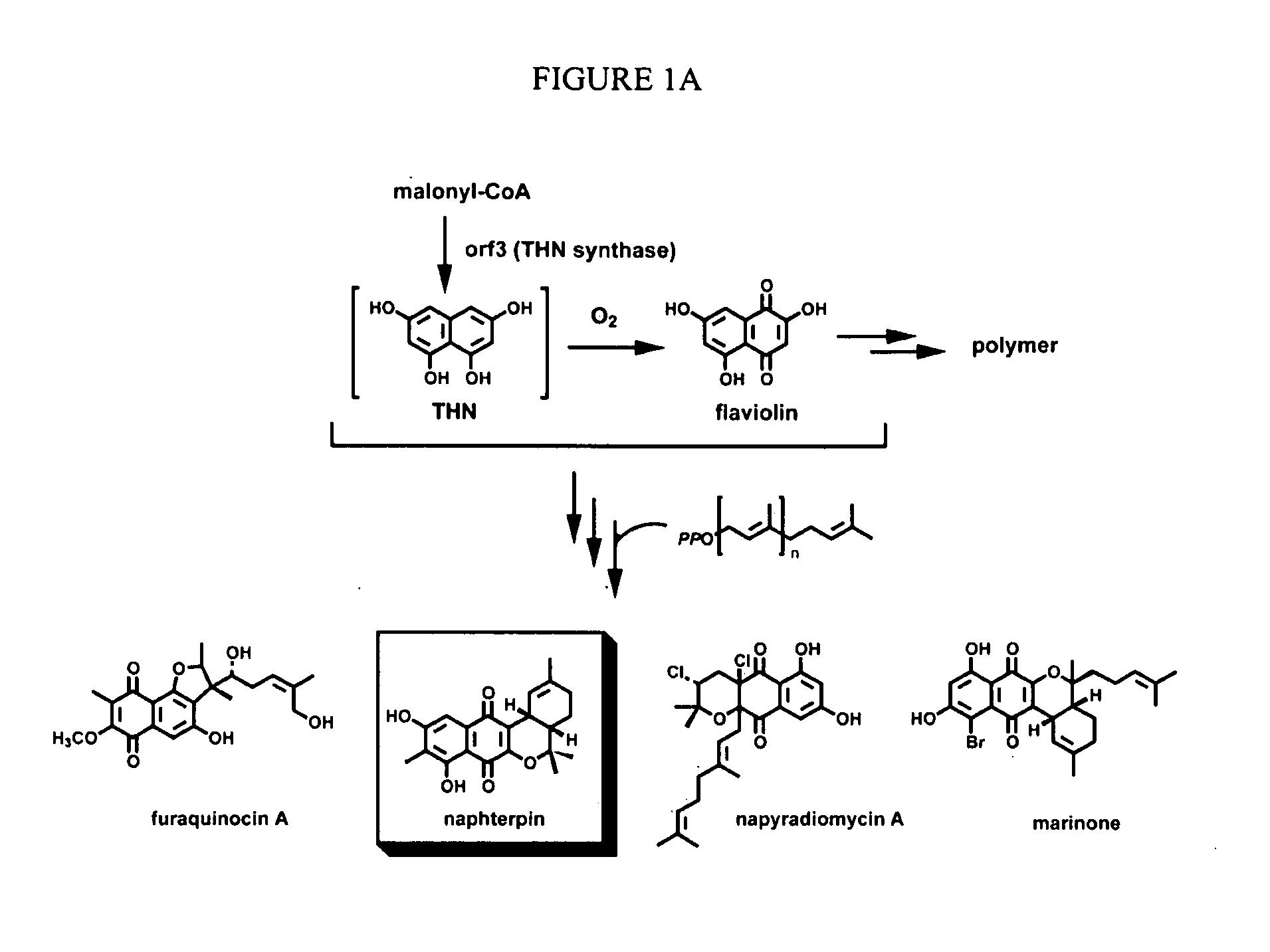
In accordance with the present invention, a novel aromatic prenyltransferase, Orf2 from Streptomyces sp. strain CL190, involved in naphterpin biosynthesis has been identified and the structure thereof elucidated. This prenyltransferase catalyzes the formation of a C—C bond between a prenyl group and a compound containing an aromatic nucleus, and also displays C—O bond formation activity. Numerous crystallographic structures of the prenyltransferase have been solved and refined, e.g., (1) prenyltransferase complexed with a buffer molecule (TAPS), (2) prenyltransferase as a binary complex with geranyl diphosphate (GPP) and Mg2+, and prenyltransferase as ternary complexes with a non-hydrolyzable substrate analogue, geranyl S-thiolodiphosphate (GSPP) and either (3) 1,6-dihydroxynaphthalene (1,6-DHN), or (4) flaviolin (i.e., 2,5,7-trihydroxy-1,4-naphthoquinone, which is the oxidized product of 1,3,6,8-tetrahydroxynaphthalene (THN)). These structures have been solved and refined to 1.5 Å, 2.25 Å, 1.95 Å and 2.02 Å, respectively. This first structure of an aromatic prenyltransferase displays an unexpected and non-canonical (β / α)-barrel architecture. The complexes with both aromatic substrates and prenyl containing substrates and analogs delineate the active site and are consistent with a proposed electrophilic mechanism of prenyl group transfer. These structures also provide a mechanistic basis for understanding prenyl chain length determination and aromatic co-substrate recognition in this structurally unique family of aromatic prenyltransferases. This structural information is useful for predicting the aromatic prenyltransferase activity of proteins.
Owner:SALK INST FOR BIOLOGICAL STUDIES
Preparation method of 2,6-dihydroxy naphthalene
InactiveCN102219651AHigh purityPrevent oxidationOrganic chemistryOrganic compound preparationAntioxidantPotassium
The invention discloses a preparation method of 2,6-dihydroxy naphthalene, which comprises the steps of: adding 98% of KOH and NaOH to a reaction kettle, heating, adding 2,6-naphthalenedisulfonic acid disodium salt and phenol, heating up to 345 to 355 DEG C and then performing reaction, cooling materials to 260 to 270 DEG C upon the completion of the reaction, adding water to dissolve the materials, then regulating an alkali fusion diluting material to a range from 1.17g / cm<3> to 1.19g / cm<3> with water, neutralizing with an sulfuric acid aqueous solution to the condition that the pH is 2 to 3, cooling, filtering and drying by pumping to obtain the crude 2,6-dihydroxy naphthalene. According to the method, a small quantity of phenol is added as an antioxidant during high temperature alkali fusion of 2,6-naphthalenedisulfonic acid disodium salt and mixed alkali, thus the generated 2,6-naphthalenediphenol sodium (potassium) is prevented from oxidation, the selectivity of the 2,6-naphthalenedisulfonic acid disodium salt is improved and the product purity is high.
Owner:NANTONG BAISHENG CHEM
Liquid-crystal polyester resin
InactiveUS6984712B2Good coloring effectImprove heat resistanceLiquid crystal compositionsLiquid crystallineHeat resistance
The present invention provides a liquid-crystalline polyester resin which comprises monomer units derived from 2-hydroxy-3-naphthoic acid and / or 2-hydroxynaphthalene-3,6-dicarboxylic acid in an amount of 1-5000 mmol % based on the total monomer components of the resin and an alkaline metal compound in an amount of 10-3000 ppm as alkaline metal based on the total monomer components of the resin. The liquid-crystalline polyester resin of the present invention has good colorability, improved heat resistance and good mechanical properties.
Owner:UENO FINE CHEM IND LTD
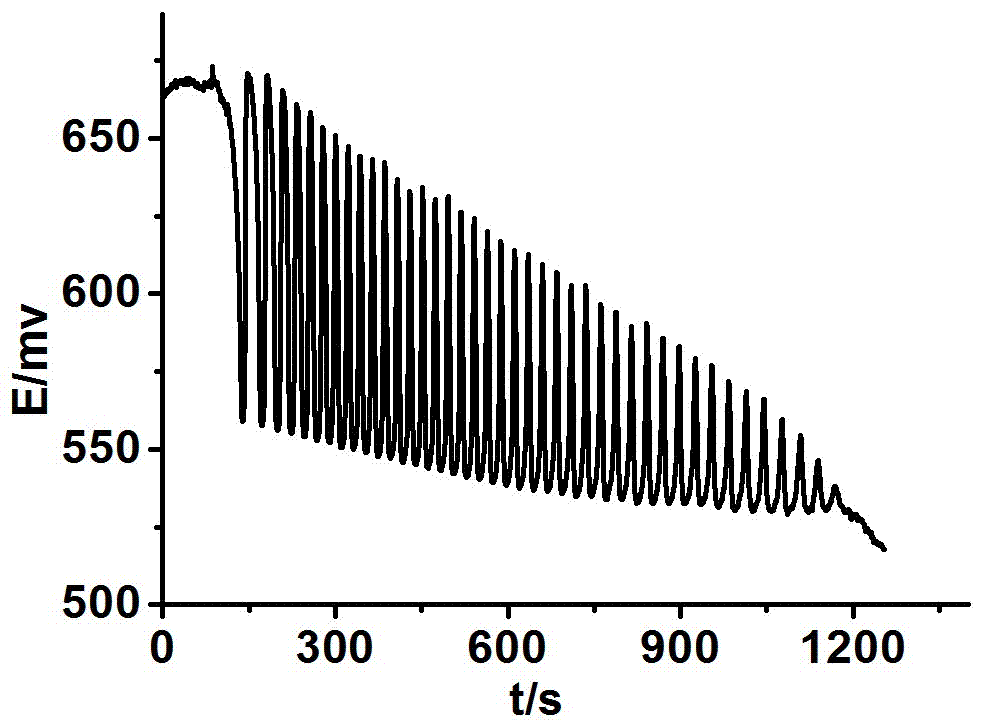
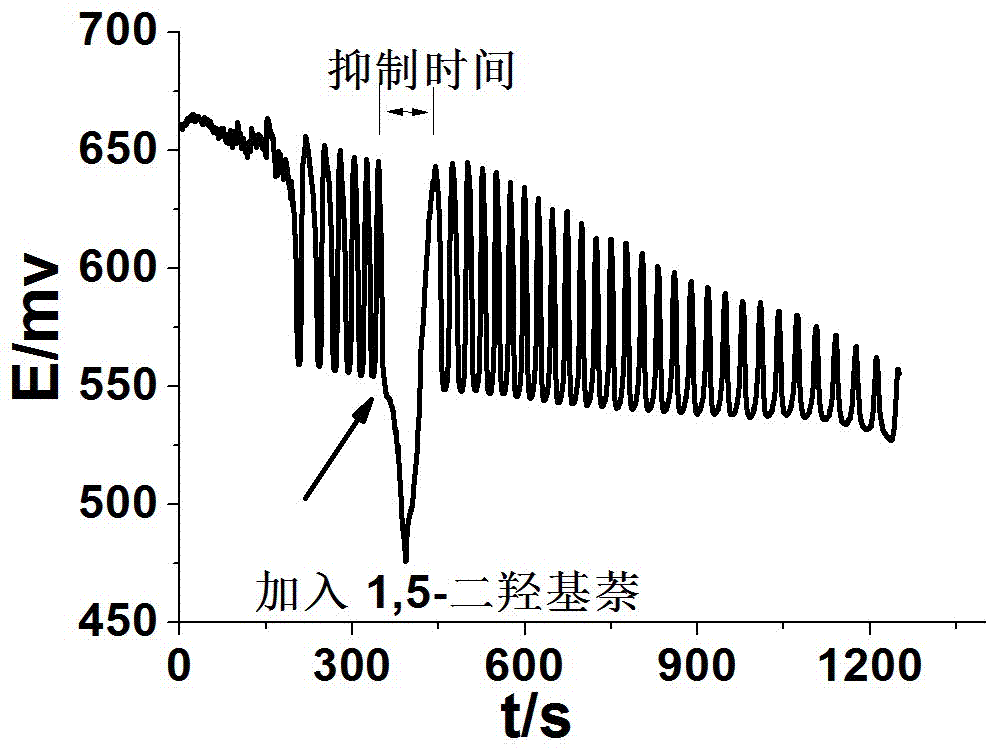
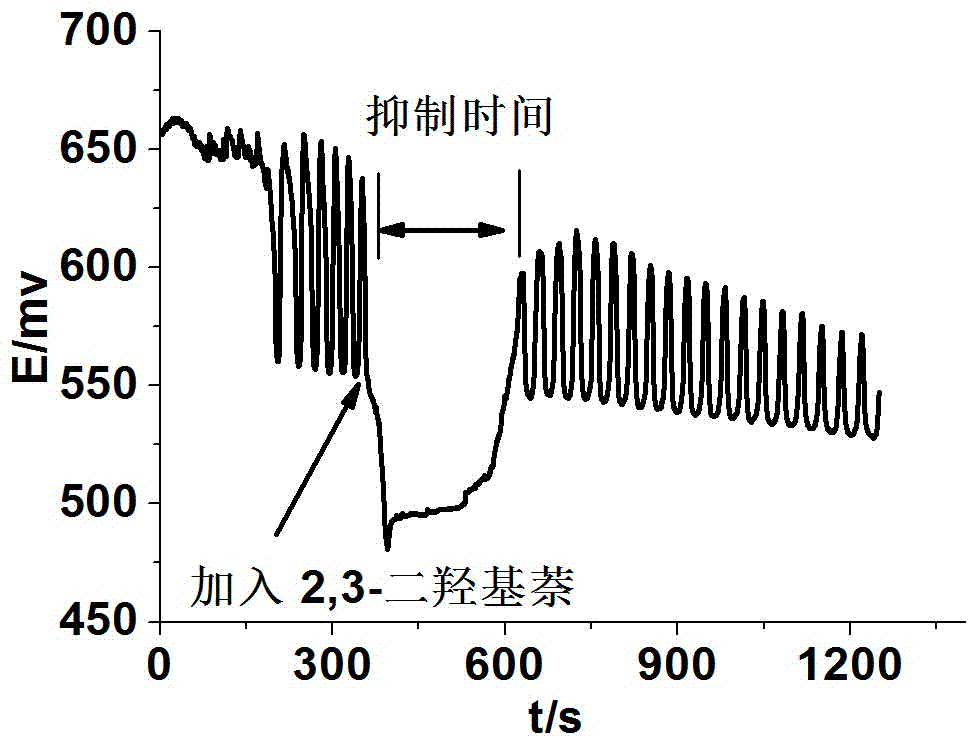
Method for identifying four kinds of dioxynaphthalene isomeride
The invention discloses a method for identifying four kinds of dioxynaphthalene isomeride. The method is characterized in that an H2SO4-KIO3-[NiL](ClO4)2-propane diacid-H2O2 nonlinear chemical oscillation system is utilized as identifying solution, and the four kinds of dioxynaphthalene isomeride can be further identified according to different oscillation responses generated by the four kinds of dioxynaphthalene isomeride of 1,5-dioxynaphthalene, 2,3-dioxynaphthalene, 2,7-dioxynaphthalene and 1,4-dioxynaphthalene. The L in the [NiL](ClO4)2 is 5,7,7,12,14,14-hexamethyl-1,4,8,11-tetraaza tetradecane-4,11-diene. An oscillation pattern provided by the identifying method has more intuition, not only can conveniently and quickly identify the four kinds of dioxynaphthalene isomeride of 1,5-dioxynaphthalene, 2,3-dioxynaphthalene, 2,7-dioxynaphthalene and 1,4-dioxynaphthalene, but also can be further widely applied to other isomeride; furthermore, the identifying method is simple in equipment and high in accuracy, and is easy to operate and observe.
Owner:ANHUI UNIVERSITY
Aromatic liquid crystal polyester and its film
An aromatic liquid-crystalline polyester having a small dielectric loss in a wide frequency region is provided. An aromatic liquid-crystalline polyester is provided that can manufacture a film having a small volume expansion by heating. An aromatic liquid-crystalline polyester substantially comprising a repeating structural unit originating in 2-hydroxy-6-naphthoic acid 30 to 80 mol %, a repeating structural unit originating in aromatic diol 35 to 10 mol %, and a repeating structural unit originating in aromatic dicarboxylic acid 35 to 10 mol %.
Owner:SUMITOMO CHEM CO LTD
Visual detection method of isothermal amplification products of nucleic acids
InactiveCN103725750ASolve pollutionRealize closed cover detectionMicrobiological testing/measurementDimerPyrophosphate
The present invention relates to two visual detection reagents of isothermal amplification products of nucleic acids, the two visual detection reagents are that 1-(1-hydroxy-2-naphthalene azo)-6-nitro-2-naphthol-4-sodium sulfonate or derivatives thereof (hereinafter referred to as the reagent 1) and 4-(2-pyridine azo) resorcinol sodium salt or derivatives thereof (hereinafter referred to as the reagent 2), the two visual detection reagents are used alone, at the beginning of a reaction, the reagent 1 combines with magnesium ions to become red, and the reagent 2 combines with the magnesium ions to form an orange complex, with the reaction, by-product pyrophosphate groups combines the magnesium ions to form a precipitation, the reagent 1 or loses the magnesium ions to become blue from red, the reagent 2 becomes yellow from orange, amplification reaction results can be judged by color change, detection without opening of a cover can be realized by the method, the aerosol pollution can be overcome, and the false positive problem caused by primer dimmers can be solved.
Owner:HARBIN DEGE BIOTECH
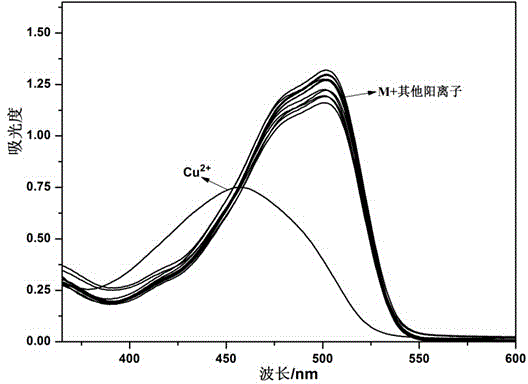
Covalent organic framework material and preparation method and application thereof in fluorescence sensor
ActiveCN110240683AHigh crystallinityHigh porosityFluorescence/phosphorescenceLuminescent compositionsCrystallinitySolvent
The invention belongs to the technical field of environmental detection, and particularly relates to a covalent organic framework material, a preparation method thereof and application thereof in a fluorescent sensor. The covalent organic framework material (COFS-DT) is obtained by reacting monomers 2,6-dihydroxynaphthalene-1,5-dialdehyde (DHNDA) and 1,3,5-tris (4-aminophenyl) benzene (TAPB) through Schiff base reaction by a solvothermal method. The crystallinity, porosity and chemical stability of the COFS-DT are improved through O-H..N = C hydrogen bond interaction, and the COFS-DT is connected with many bidentate coordination sites interacting with target ions. Compared with the monomers, the COFS-DT shows stronger fluorescence when dispersed in isopropanol. The strong fluorescence and the bidentate coordination sites enable the COFS-DT to be used to construct a high-performance fluorescence sensor with high sensitivity, high selectivity and high stability for detecting Cu<2+>.
Owner:SHANDONG UNIV
Fluorescent molecular probe for detecting fluoride ions as well as synthesis method and application thereof
InactiveCN104418875AHigh sensitivityStable fluorescenceGroup 4/14 element organic compoundsMaterial analysis by observing effect on chemical indicatorHigh fluoridePhosphate
The invention relates to a preparation method of a fluorescent molecular probe for detecting fluoride ions through colorimetric detection and fluorescence enhancement and an application of the fluorescent molecular probe to detecting fluoride ions. The fluorescent molecular probe is prepared by protecting 2-hydroxyl-1-naphthaldehyde taken as a raw material with silane and then condensing the raw material and malononitrile. The fluorescent molecular probe is simple and convenient to synthesize, and reaction conditions are mild. The fluorescent molecular probe has the specific characteristics that the probe molecule has stable optical properties and higher synthetic yield; the probe molecule has high fluoride ion detection sensitivity and low lower limit of detection, and the limit of detection is 0.52mu M; the response range is 0-100mu M and the detection range is wide; the probe molecule has good selectivity and has no responses to anions, such as dihydrogen phosphate radicals, acetate radicals, bromide ions, hydrosulfate radicals, chlorate radicals, iodide ions, chloride ions and nitrate radicals; the probe molecule is suitable for colorimetric detection; the fluorescent molecular probe has practical application values in the fields of biochemistry, environmental sciences and the like.
Owner:SUZHOU ROWLAND BIOTECH
Low-temperature-resistant rare earth ore flotation collecting agent and preparation method and application thereof
The invention discloses a low-temperature-resistant rare earth ore flotation collecting agent. The collecting agent comprises, by weight, 30-40 parts of 2-hydroxybenzohydroxamic acid, 30-50 parts of C5-7 hydroxamic acid, 5-15 parts of o-methyl naphthalene hydroxamic acid, 5-10 parts of coconut oil acid and 1-2.5 parts of surface active agents. The invention further provides a preparation method of the collecting agent. The collecting agent is good in water solubility, resistant to low temperature, excellent in selectivity and collecting property, good in flotation performance and easy to degrade, and the defects that an existing collecting agent for industrial application is poor in selectivity and difficult to degrade and needs heating flotation are overcome; and the production technique of the prepared collecting agent is simple and easy to implement, capital construction investment and energy consumption are reduced for enterprises, and the profit of the enterprises is increased.
Owner:INST OF MULTIPURPOSE UTILIZATION OF MINERAL RESOURCES CHINESE ACAD OF GEOLOGICAL SCI
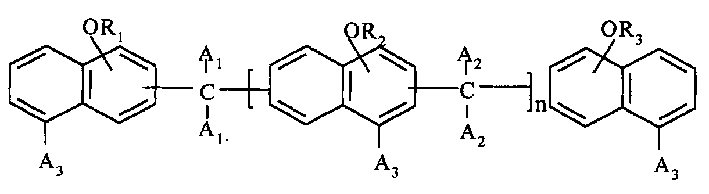
Polyfunctional epoxy resin and its preparation method
A kind of multifunctinoal epoxy resin and its preparation method is involved in this invention. In the formula, A3 is selected from hydrogen atom, cymene, methoxy, bromine atom, R1, R2, R3 are shrink glycerin aethers, the value of n lies between 0 and 8. The preparation process of the method includes: firstly, hydroxy naphthalene compound and bifunctional aldehyde or ketone shrink in the presence of proton acid activator, and multihydroxy naphthalene compound is gained, and then it makes aether reaction with PAE in the action of alkali activator. The epoxy resin has good capability such as high heat-resistance, low moisture absorption and low expandability etc., and fits madding glass cloth or epoxy resin pressing board covering copper and foil, and encapsulation of semiconductor component and IC, so it has capacious application foreground in the electronic industry.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Use of 2,3-dihydroxynaphthalene-6-sulfonic acid salts as dispersants
Stable dispersions of nanoparticles and microparticles in liquids and method for their preparation are disclosed. The dispersions comprise about 0.1 wt % to about 25 wt % of a 2,3-dihydroxynaphthalene-6-sulfonic acid salt or mixture of salts of 2,3-dihydroxynaphthalene-6-sulfonic acid; about 1 wt % to about 90 wt % of the particles; and about 10 wt % to about 90 wt % of the liquid.
Owner:AIR PROD & CHEM INC
Organic fluorescent gel compound based on naphthalimide and preparation method and application thereof
InactiveCN103992271AAchieving Sensitive DetectionOrganic chemistryFluorescence/phosphorescenceImideChemical compound
The invention discloses an organic fluorescent gel compound based on naphthalimide. The compound is alkyl chain carboxylic acid 4-hydroxyl-N-caproyl-1,8-naphthalimide with 4-hydroxy naphthalimide as a chromophore, and has a structural formula as below, and a molecular formula of [6-HO-C12H5NO2-(CH2)5-COOH]. The invention uses simple fatty acids to introduce the naphthalimide group with fluorescence property, and provides a novel simple gel system; at the same time, the gelator has response function to triethylamine, addition of triethylamine results in fluorescence quenching, and addition of a certain amount of acid generates fluorescence recovery, therefore the invention uses this property, to realize sensitive detection of tri ethylamine by the gel system.
Owner:XINYANG NORMAL UNIVERSITY
Controlled release composition and method of producing the same
InactiveUS7429559B2Suppressing initial excess releaseStable release rateNervous disorderPeptide/protein ingredientsControl releaseMedicine
A controlled release composition containing a physiologically active substance in high content, suppressing the initial excess release, and achieving a stable release speed over a long period of time is provided.A controlled release composition comprising (1) a physiologically active substance or salt thereof in an amount of about 14% (w / w) to about 24% (w / w) based on the total composition weight, (2) hydroxynaphthoic acid selected from the group consisting of 3-hydroxy-2-naphthoic acid and 1-hydroxy-2-naphthoic acid or salt thereof, and (3) a lactic acid polymer or salt thereof having a weight-average molecular weight of 15000 to 50000 in which the content of polymers having molecular weights of 5000 or less is about 5% by weight or less, wherein the molar ratio of said hydroxynaphthoic acid or salt thereof to said physiologically active substance or salt thereof is from 3:4 to 4:3.
Owner:TAKEDA PHARMA CO LTD
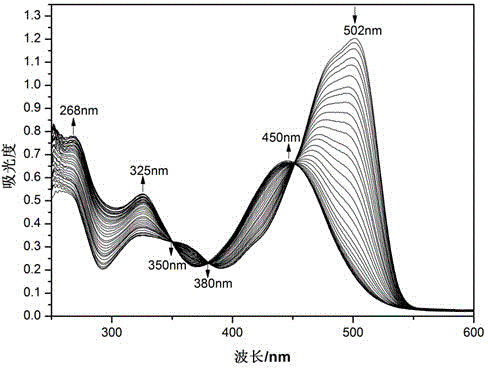
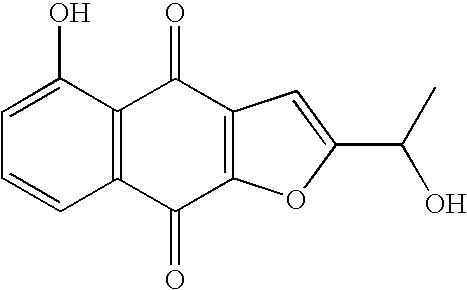
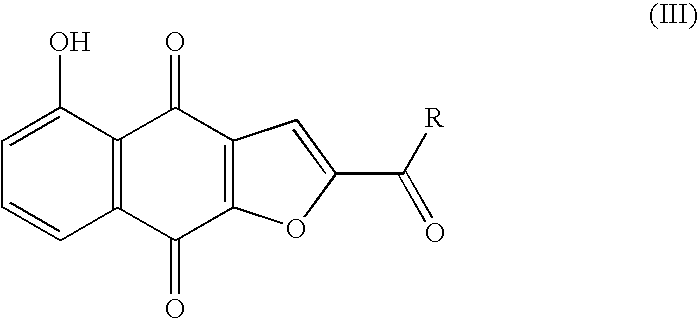
Preparation of optically active 2-(1-hydroxyethyl)-5-hydroxynaphtho[2,3-b]furan-4, 9-diones having anticancer activities
An object of the present invention is to provide a method for efficiently preparing (S)-2-(1-hydroxyethyl)-5-hydroxynaphtho[2,3-b]-furan-4,9-dione useful as a medicine at a low cost and in large amounts. According to the present invention, the desired (S)-2-(1-hydroxyethyl)-5-hydroxynaphtho[2,3-b]furan-4,9-dione can be prepared with high efficiency at a low cost and in large amounts by asymmetrically reducing 2-acetyl-5-hydroxynaphtho[2,3-b]furan-4,9-dione in the presence of an asymmetric ruthenium complex and a hydrogen donor.
Owner:TAHEEBO JAPAN
Wholly aromatic liquid crystalline polyester resin, molded article, and electric and electronic components
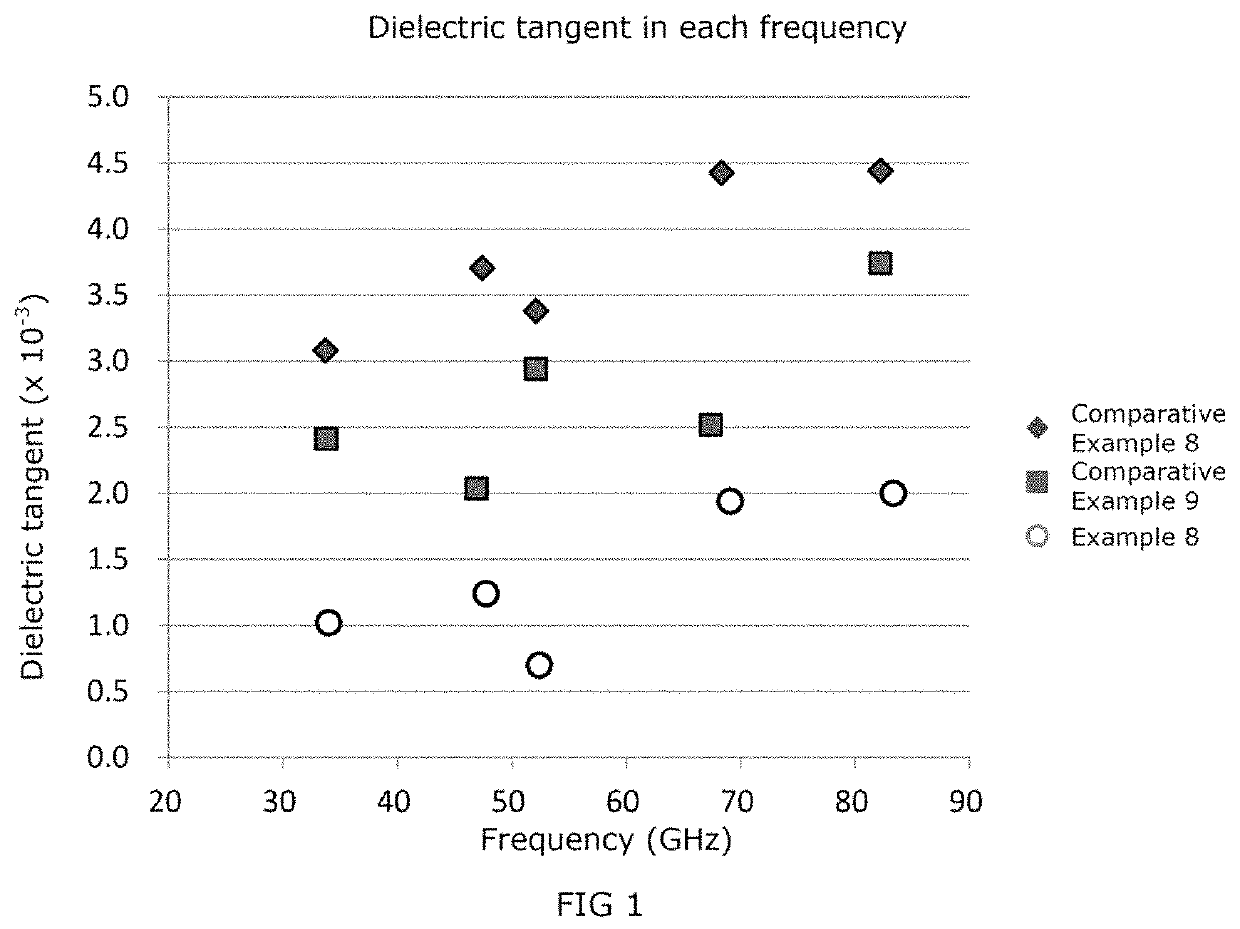
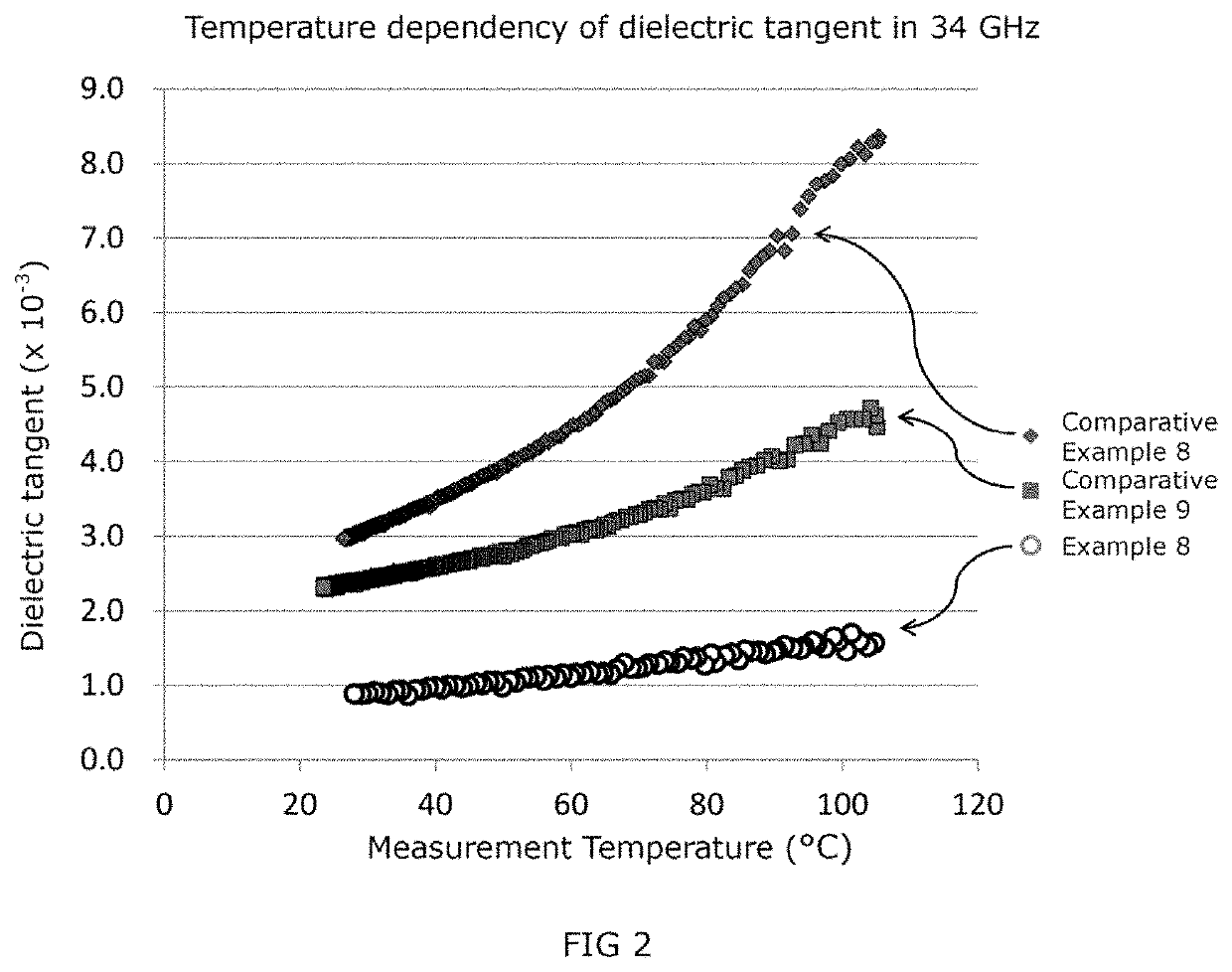
ActiveUS20200040133A1Low dielectric tangentProlonged heat treatmentCoatingsLiquid crystallineBenzoic acid
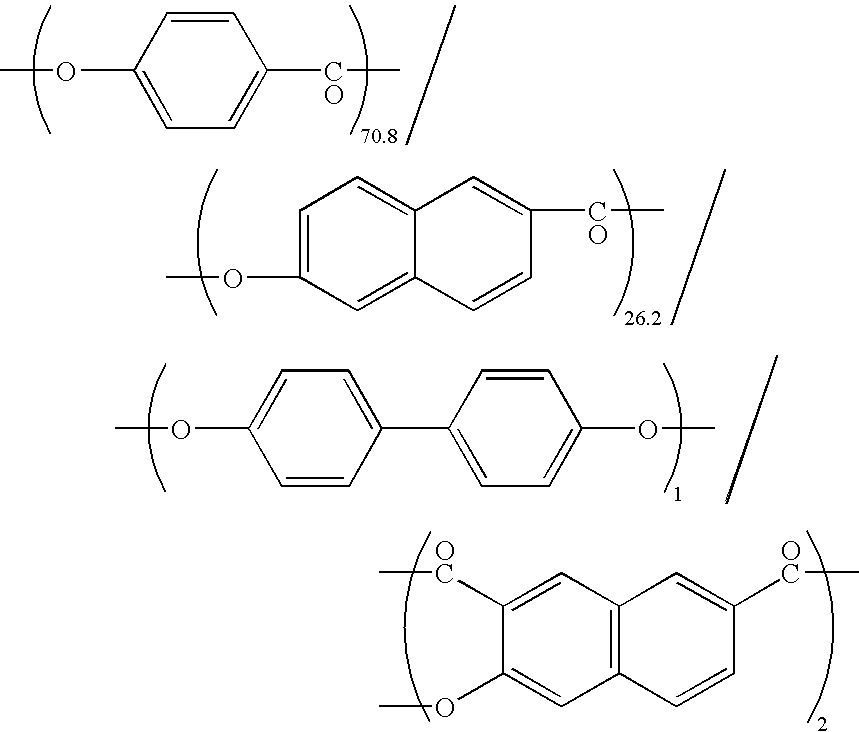
[Problem] To provide a wholly aromatic liquid crystalline polyester resin having an excellent heat resistance and processability while having an extremely low dielectric tangent[Solving means] A wholly aromatic liquid crystalline polyester resin according to the present invention comprises,structural unit (I) derived from p-hydroxybenzoic acid,structural unit (II) derived from 6-hydroxy-2-naphthoic acid,structural unit (III) derived from an aromatic diol compound,structural unit (IV) derived from an aromatic dicarboxylic acid, whereinthe composition ratio (mol %) of said structural units (I) to (IV) satisfies the following conditions:2 mol %≤structural unit (I)≤9 mol %40 mol %≤structural unit (II)≤75 mol %9 mol %≤structural unit (III)≤24 mol %9 mol %≤structural unit (IV) 24 mol %.
Owner:ENEOS CORP
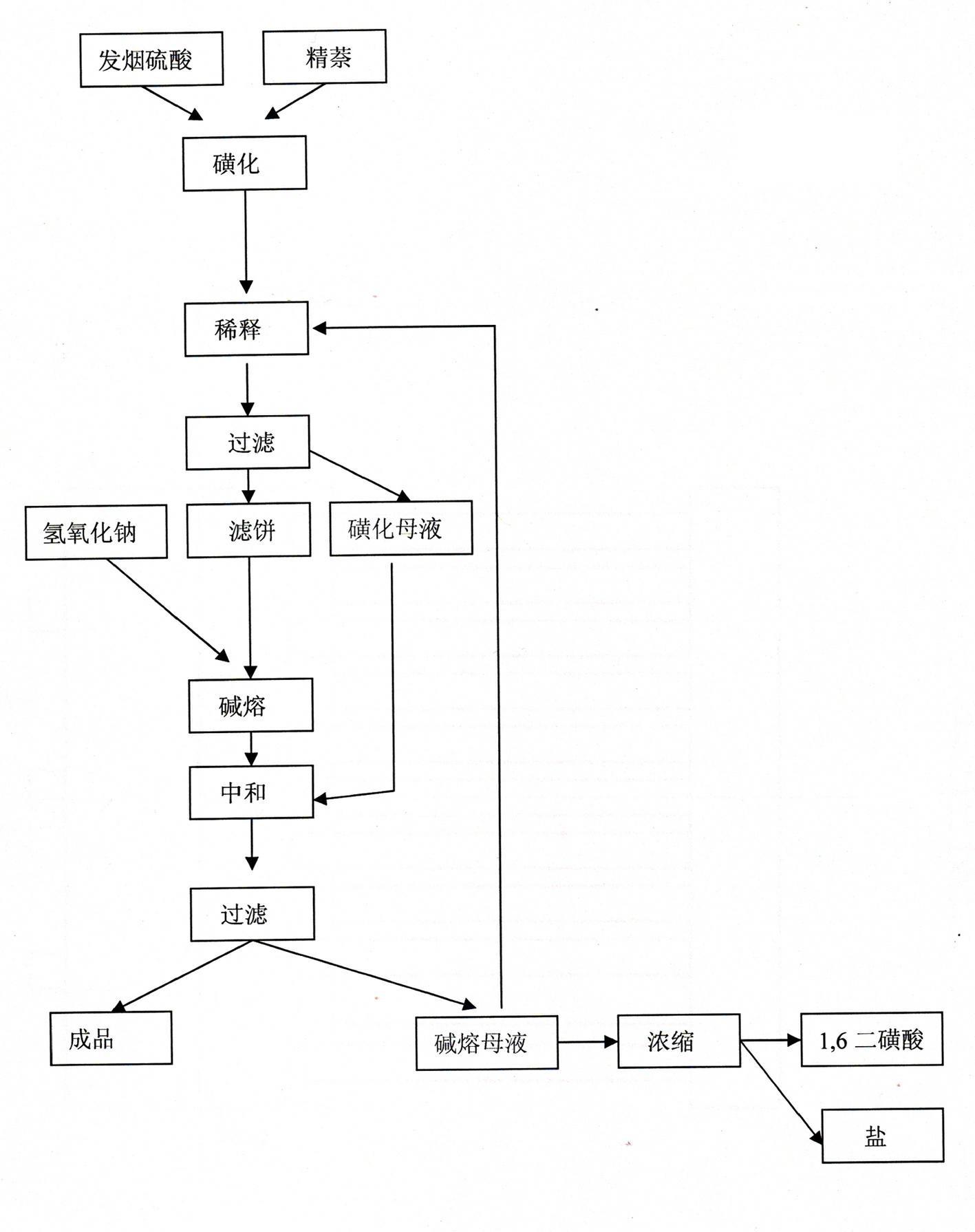
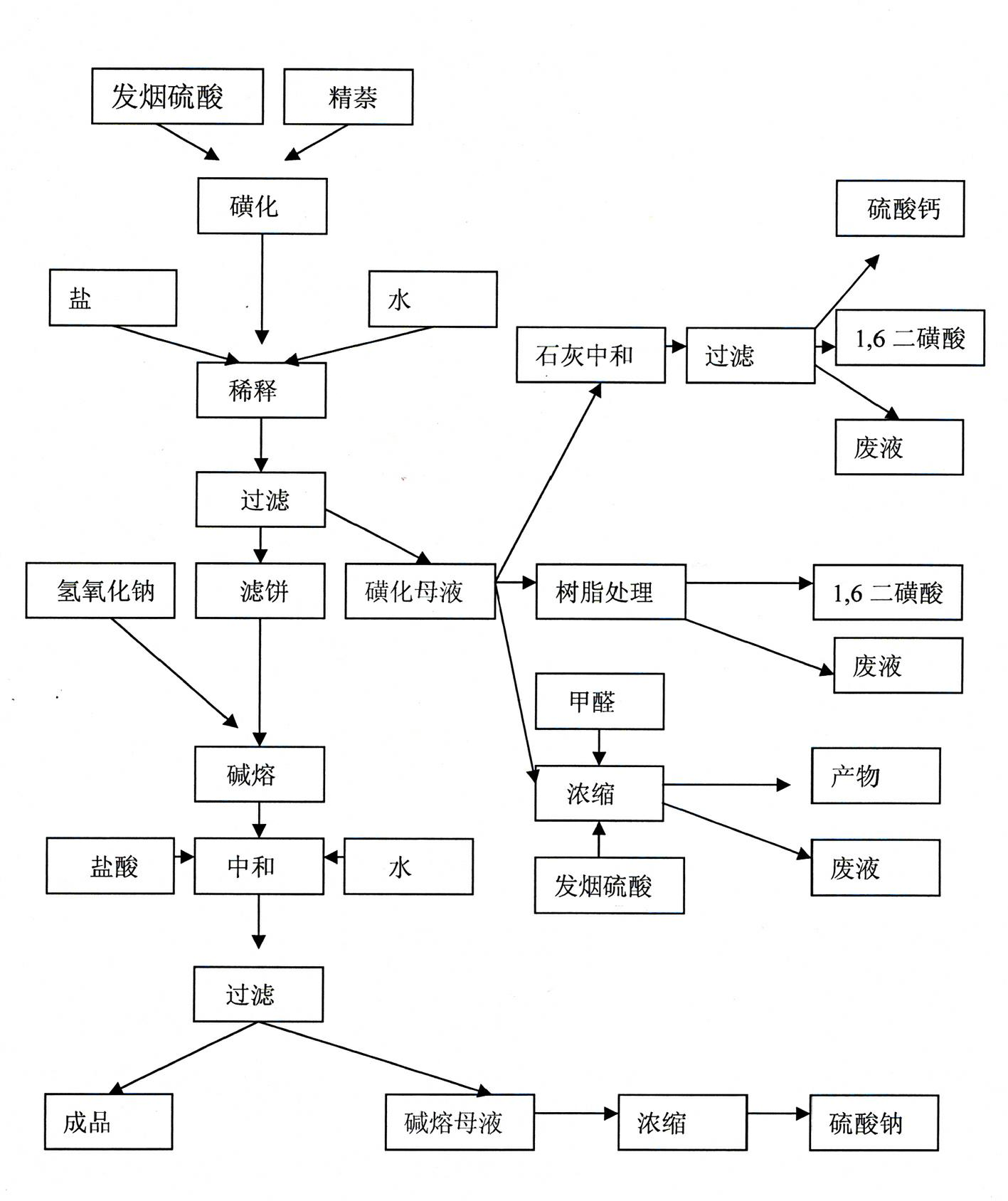
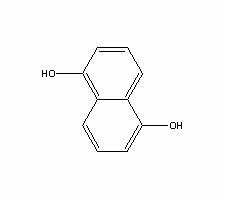
Method for producing 1,5-dihydroxy naphthalene
InactiveCN102442888AEasy to handleReduce joinOrganic compound preparationSulfonic acid preparationHydroxy groupSulphate sodium
The invention discloses a technological method for producing 1,5-dihydroxy naphthalene by a sulfonating alkali fusion method. The technological method comprises the steps of: 1, sulfonating; 2, thinning, after secondary treatment, thinning by using sodium sulfate solution obtained in a step 7; 3, filtering; 4, fusing by alkali; 5, neutralizing, adding sulfonation mother liquor obtained in the step 3 into alkali fusion product for neutralization; 6, filtering; and 7, sending partial alkali fusion mother liquor to the step 2 and thinning the same, and treating the rest alkali fusion mother liquor by alkali fusion. By using the mother liquor of the production progress, the total amount of the mother liquor is reduced, the total amount of waste water is decreased, and treatment difficulty of waste water is lowered.
Owner:DONDONG SUNLINE CHEM
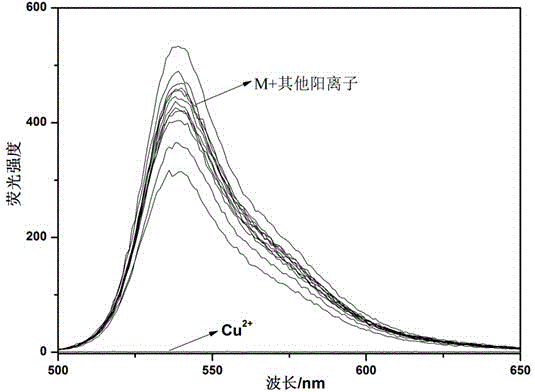
Two-sided Schiff base sensor, synthesis method thereof, and application thereof in fluorescent colorimetric continuous identification of Cu<2+> and H<2>PO<4><->
InactiveCN104914079AMaterial analysis by observing effect on chemical indicatorFluorescence/phosphorescenceDianisidineFluorophore
The invention discloses a two-sided Schiff base sensor molecule based on dianisidine and 2-hydroxy-1-naphthaldehyde. The two-sided Schiff base sensor molecule has a fluorophore naphthalene ring structure, the naphthalene ring has -OH, the Schiff base has a -C=N- structure, and metal ions Cu<2+> can easily coordinate with N and O atoms to form a complex, so the two-sided Schiff base sensor molecule can identify Cu<2+>. H<2>PO<4><-> is added to a complex formed by the sensor molecule M and Cu<2+> to make Cu<2+> and H<2>PO<4><-> form a more stable complex, so Cu<2+> is substituted in am acceptor, thereby Cu<2+> and H<2>PO<4><-> can be continuously identified. The sensor molecule can conveniently, rapidly and efficiently detect Cu<2+> and H<2>PO<4><-> in an aqueous system, and the sensor molecule can be switched into different fluorescence emission states comprising opening (strong fluorescence emission) or closure (fluorescence quenching), so the sensor molecule can also be applied in the field of molecule switches.
Owner:NORTHWEST NORMAL UNIVERSITY
Anticancer compound, intermediate therefor, and processes for producing these
The present invention provides a method for easily and inexpensively preparing a racemate or an optically-active 2-(1 -hydroxyethyl)-5-hydroxynaphtho[2,3-b]furan-4,9-dione in high yields, 2-acetyl-2,3-dihydro-5-hydroxynaphtho[2,3-b]furan-4,9-dione which is useful as an intermediate for preparing NFD, and an anticancer agent comprising 2-(1-hydroxyethyl)-5-hydroxynaphtho[2,3-b]furan-4,9-dione as an active ingredient.Said 2-(1-hydroxyethyl)-5-hydroxynaphtho[2,3-b]furan-4,9-dione is obtained in 4 or 5 steps by using comparatively inexpensive 5 -hydroxynaphthalene-1,4-dione (also referred to as juglone) as a starting material.
Owner:TAHEEBO JAPAN
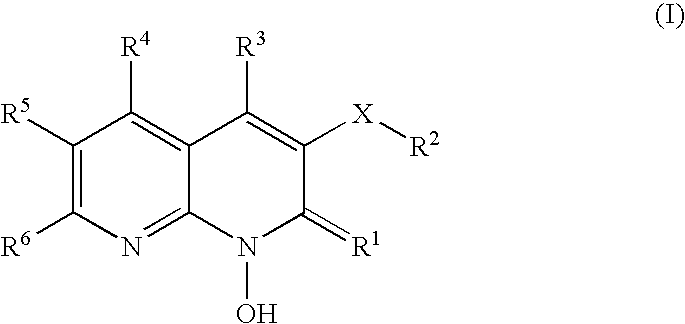
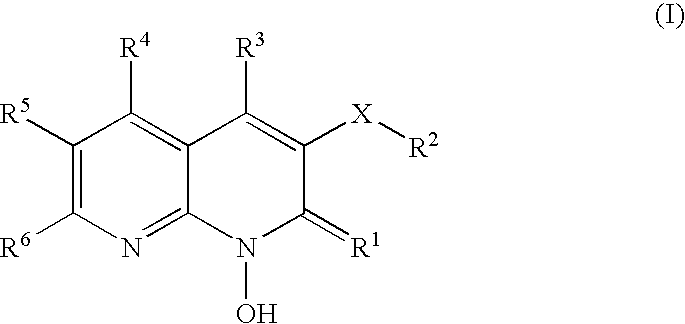
1-hydroxy naphthyridine compounds as Anti-hiv agents
1-Hydroxy naphthyridine compounds (e.g., 1-hydroxy naphthyridin-2(1H)-one compounds of Formula I are inhibitors of HIV integrase and / or HIV RNase H and inhibitors of HIV replication: (I) wherein X and R1-R6 are as defined herein. The compounds are useful in the prophylaxis and treatment of infection by HIV and in the prophylaxis, delay in the onset, and treatment of AIDS. The compounds are employed against HIV infection and AIDS as compounds per se or in the form of pharmaceutically acceptable salts. The compounds and their salts can be employed as ingredients in pharmaceutical compositions, optionally in combination with other anti-HIV agents such as HIV antivirals, immunomodulators, antibiotics and vaccines.
Owner:MERCK SHARP & DOHME CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
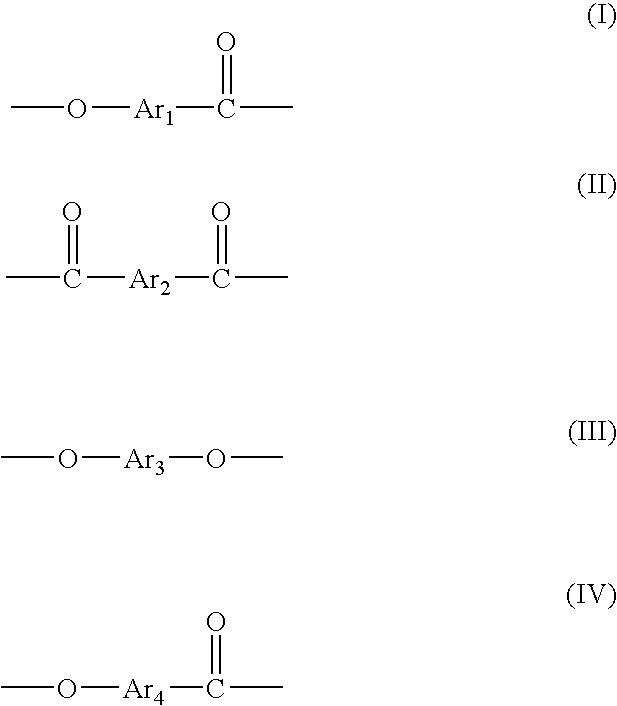
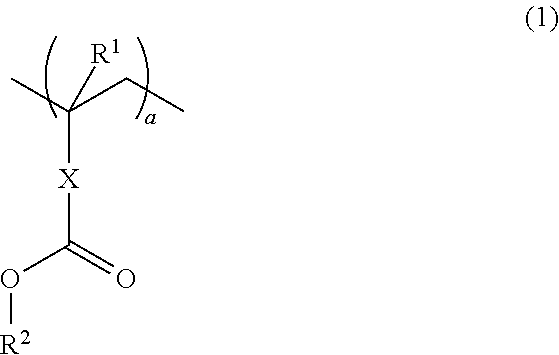
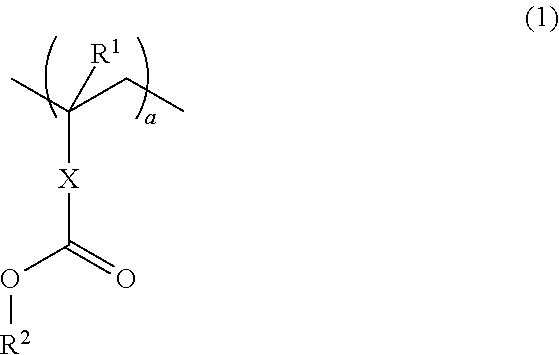
![Pillar[5]arene and 2-hydroxy-3-naphthoic acid complex and preparation thereof and application in detecting iron ions and fluorine ions Pillar[5]arene and 2-hydroxy-3-naphthoic acid complex and preparation thereof and application in detecting iron ions and fluorine ions](https://images-eureka.patsnap.com/patent_img/9701a422-70b5-4870-9f49-92bcf764c7de/160401111727.PNG)
![Pillar[5]arene and 2-hydroxy-3-naphthoic acid complex and preparation thereof and application in detecting iron ions and fluorine ions Pillar[5]arene and 2-hydroxy-3-naphthoic acid complex and preparation thereof and application in detecting iron ions and fluorine ions](https://images-eureka.patsnap.com/patent_img/9701a422-70b5-4870-9f49-92bcf764c7de/160401111731.PNG)
![Pillar[5]arene and 2-hydroxy-3-naphthoic acid complex and preparation thereof and application in detecting iron ions and fluorine ions Pillar[5]arene and 2-hydroxy-3-naphthoic acid complex and preparation thereof and application in detecting iron ions and fluorine ions](https://images-eureka.patsnap.com/patent_img/9701a422-70b5-4870-9f49-92bcf764c7de/160401111735.PNG)
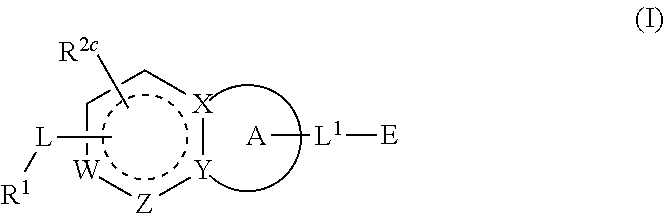
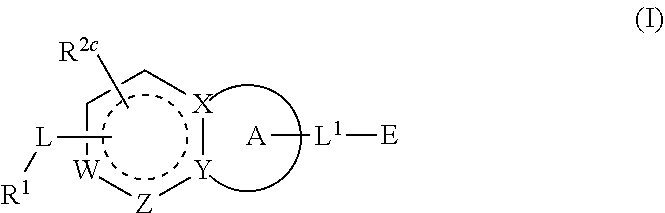


![Preparation of optically active 2-(1-hydroxyethyl)-5-hydroxynaphtho[2,3-b]furan-4, 9-diones having anticancer activities Preparation of optically active 2-(1-hydroxyethyl)-5-hydroxynaphtho[2,3-b]furan-4, 9-diones having anticancer activities](https://images-eureka.patsnap.com/patent_img/a2e895ec-d7af-426f-8bf7-f1525fcc9571/US20080300415A1-20081204-C00001.png)
![Preparation of optically active 2-(1-hydroxyethyl)-5-hydroxynaphtho[2,3-b]furan-4, 9-diones having anticancer activities Preparation of optically active 2-(1-hydroxyethyl)-5-hydroxynaphtho[2,3-b]furan-4, 9-diones having anticancer activities](https://images-eureka.patsnap.com/patent_img/a2e895ec-d7af-426f-8bf7-f1525fcc9571/US20080300415A1-20081204-C00002.png)
![Preparation of optically active 2-(1-hydroxyethyl)-5-hydroxynaphtho[2,3-b]furan-4, 9-diones having anticancer activities Preparation of optically active 2-(1-hydroxyethyl)-5-hydroxynaphtho[2,3-b]furan-4, 9-diones having anticancer activities](https://images-eureka.patsnap.com/patent_img/a2e895ec-d7af-426f-8bf7-f1525fcc9571/US20080300415A1-20081204-C00003.png)