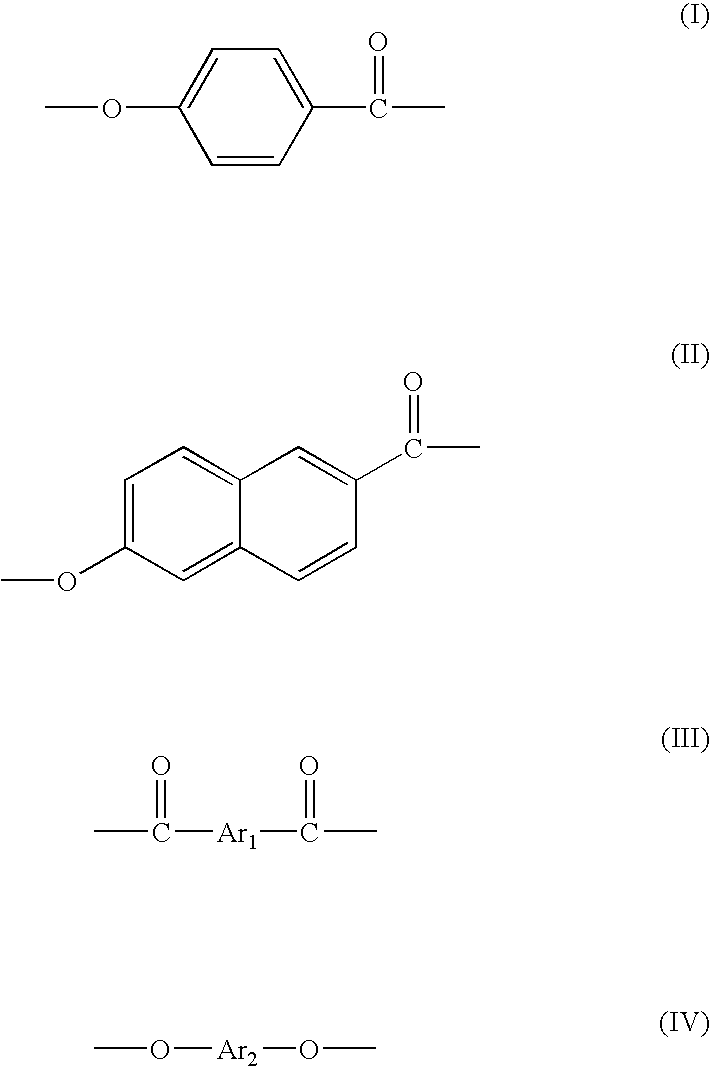
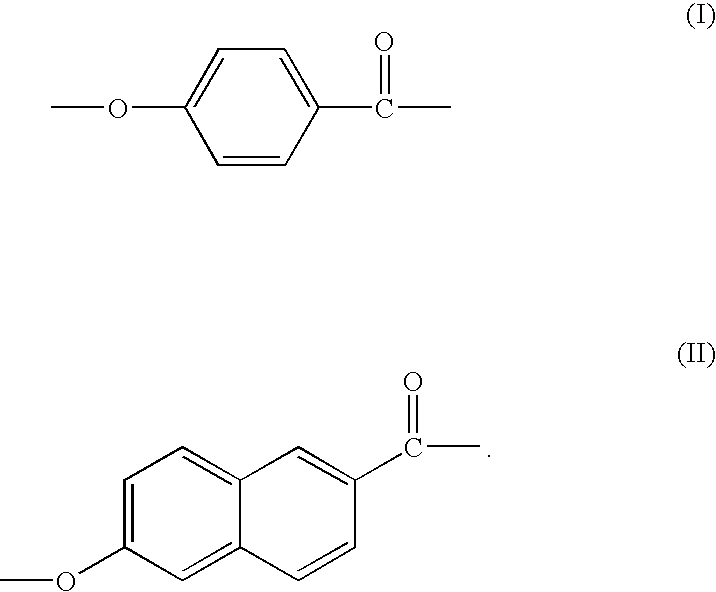
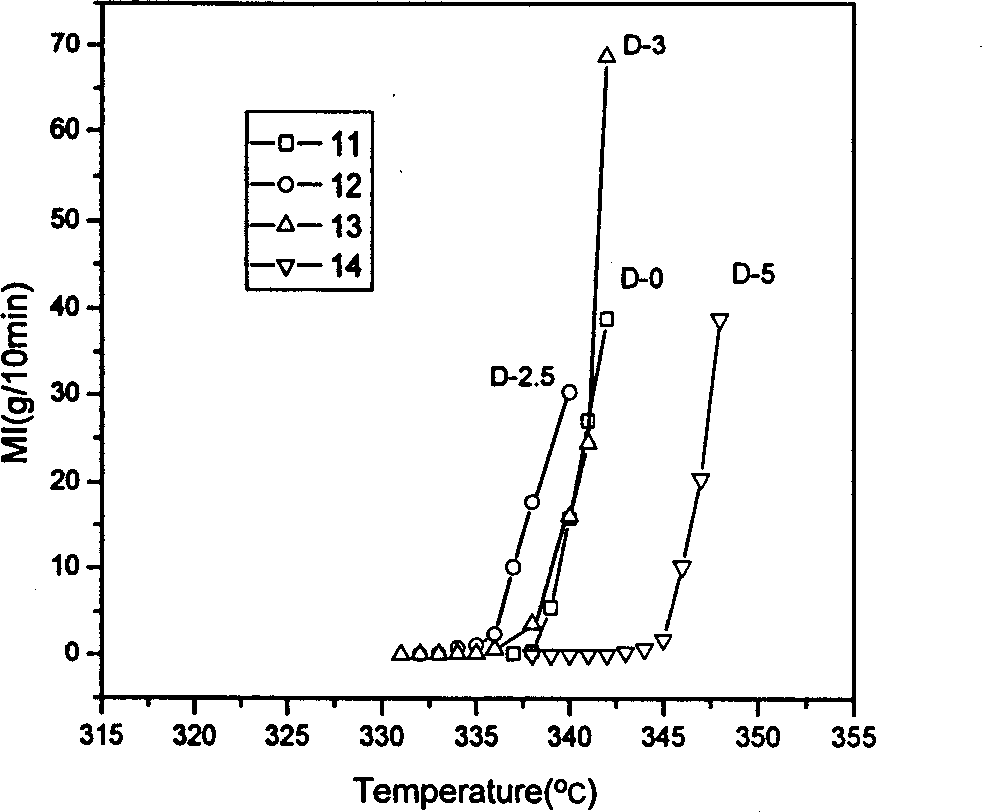
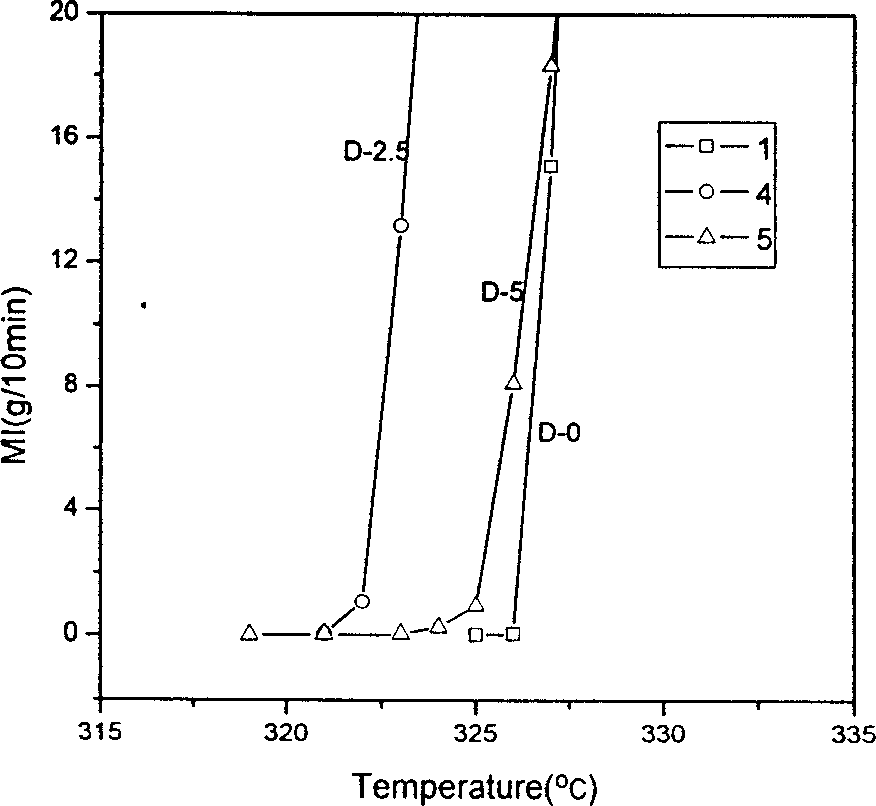
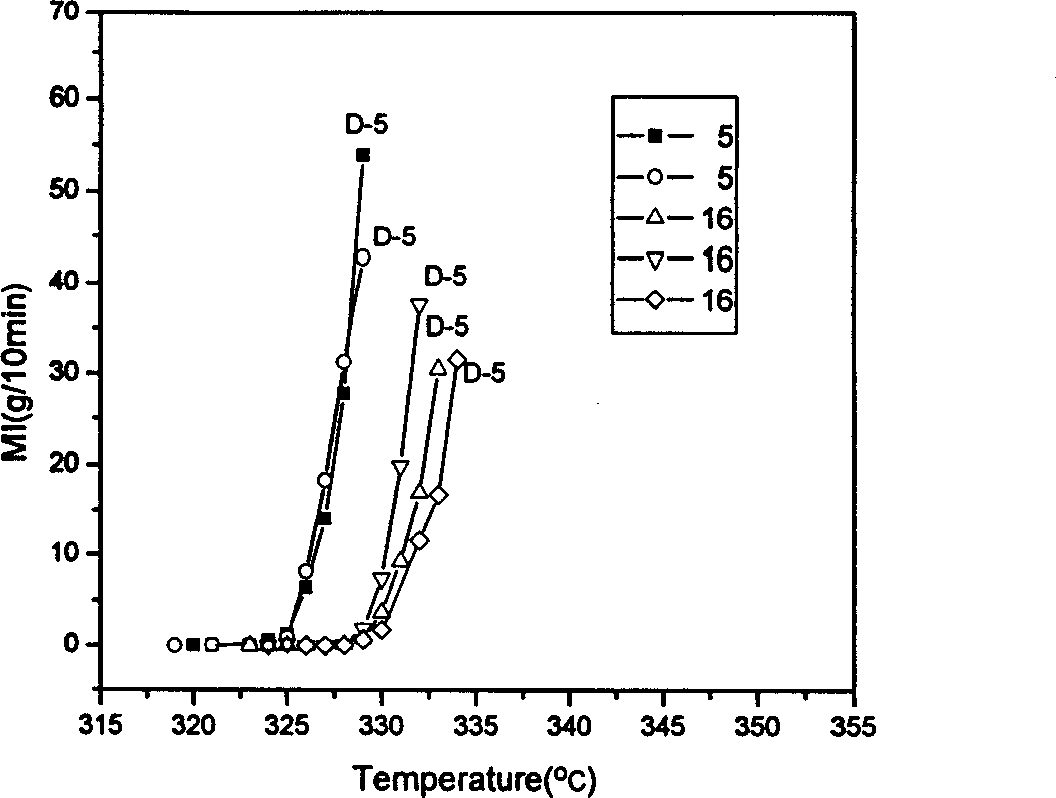
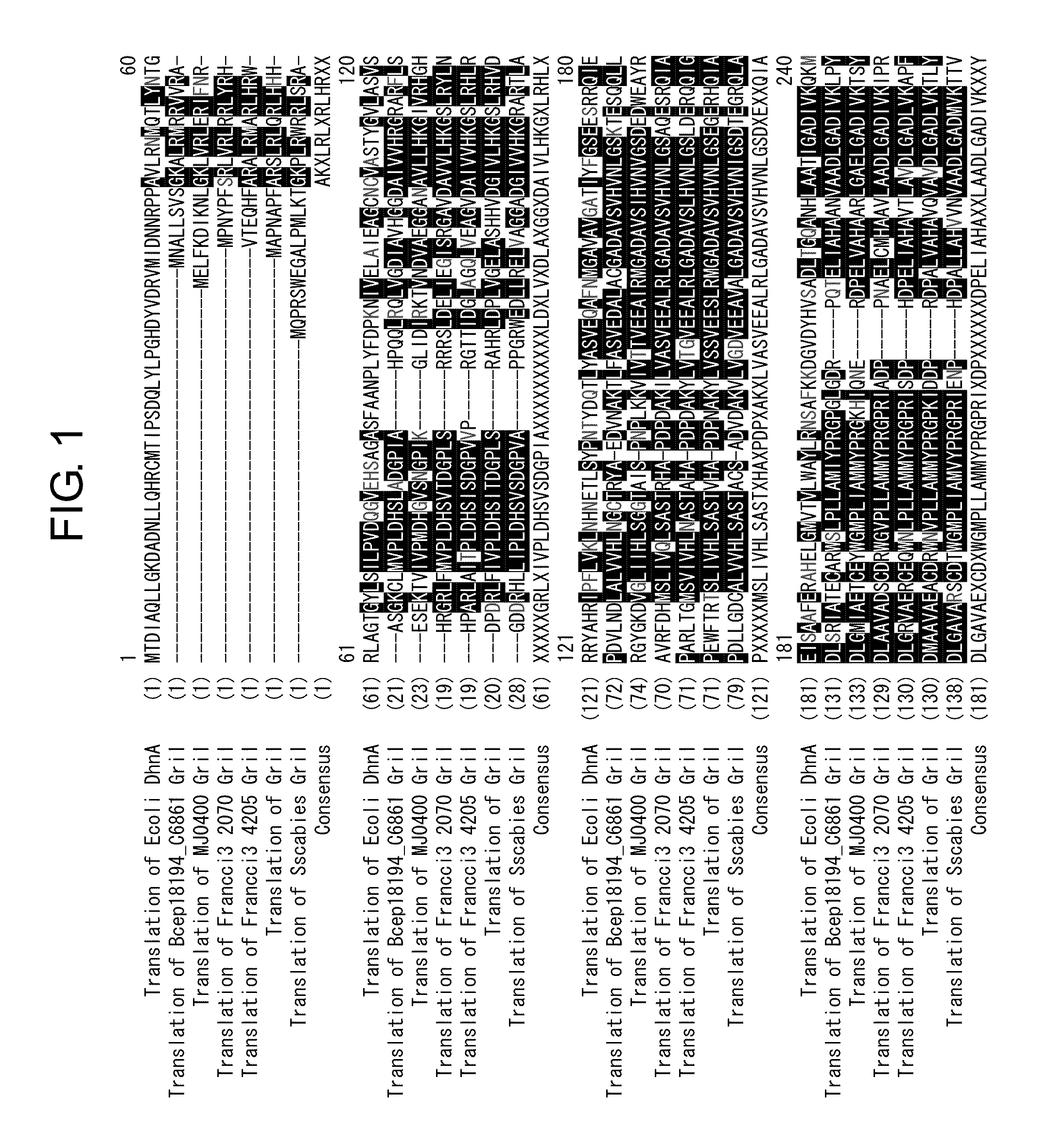
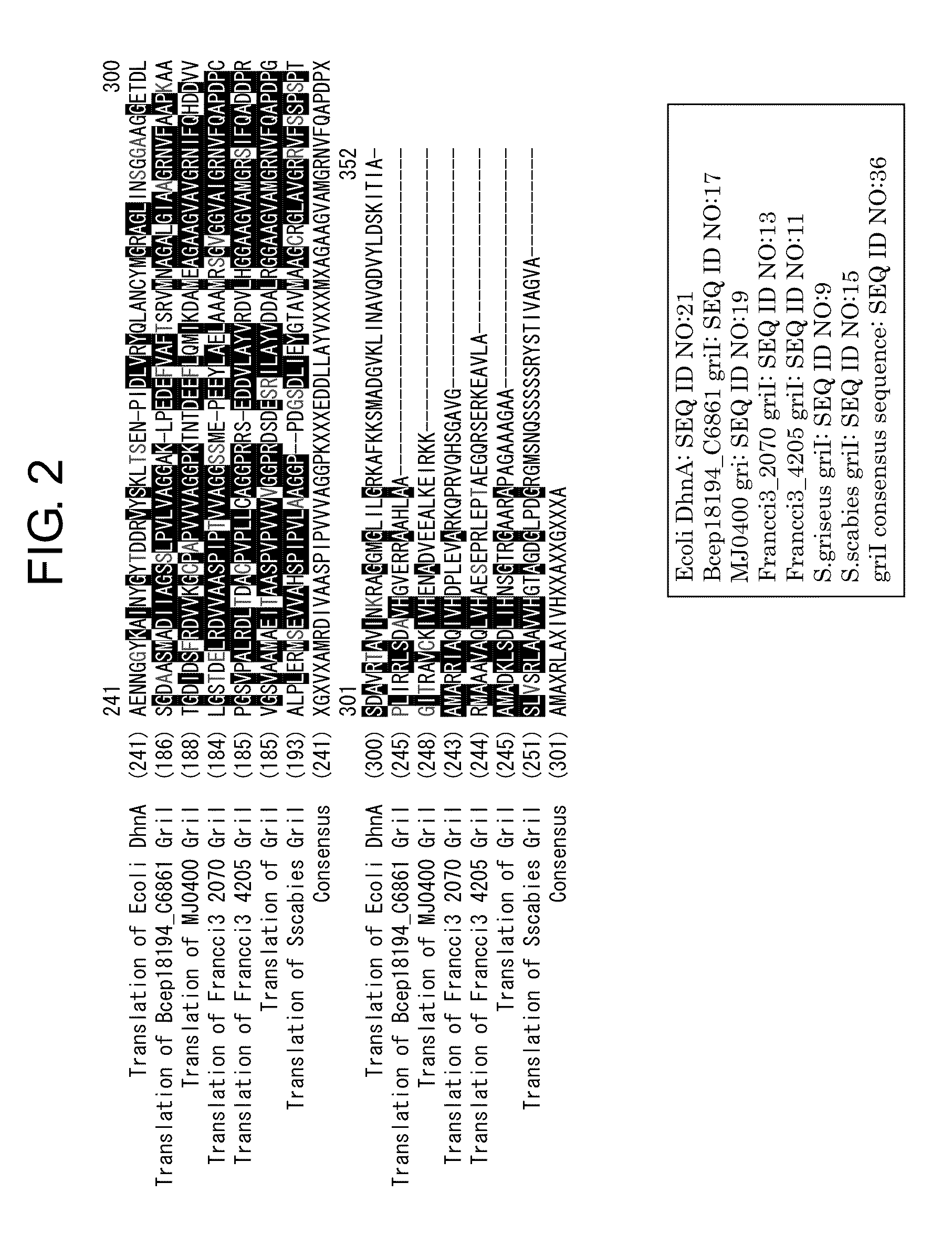
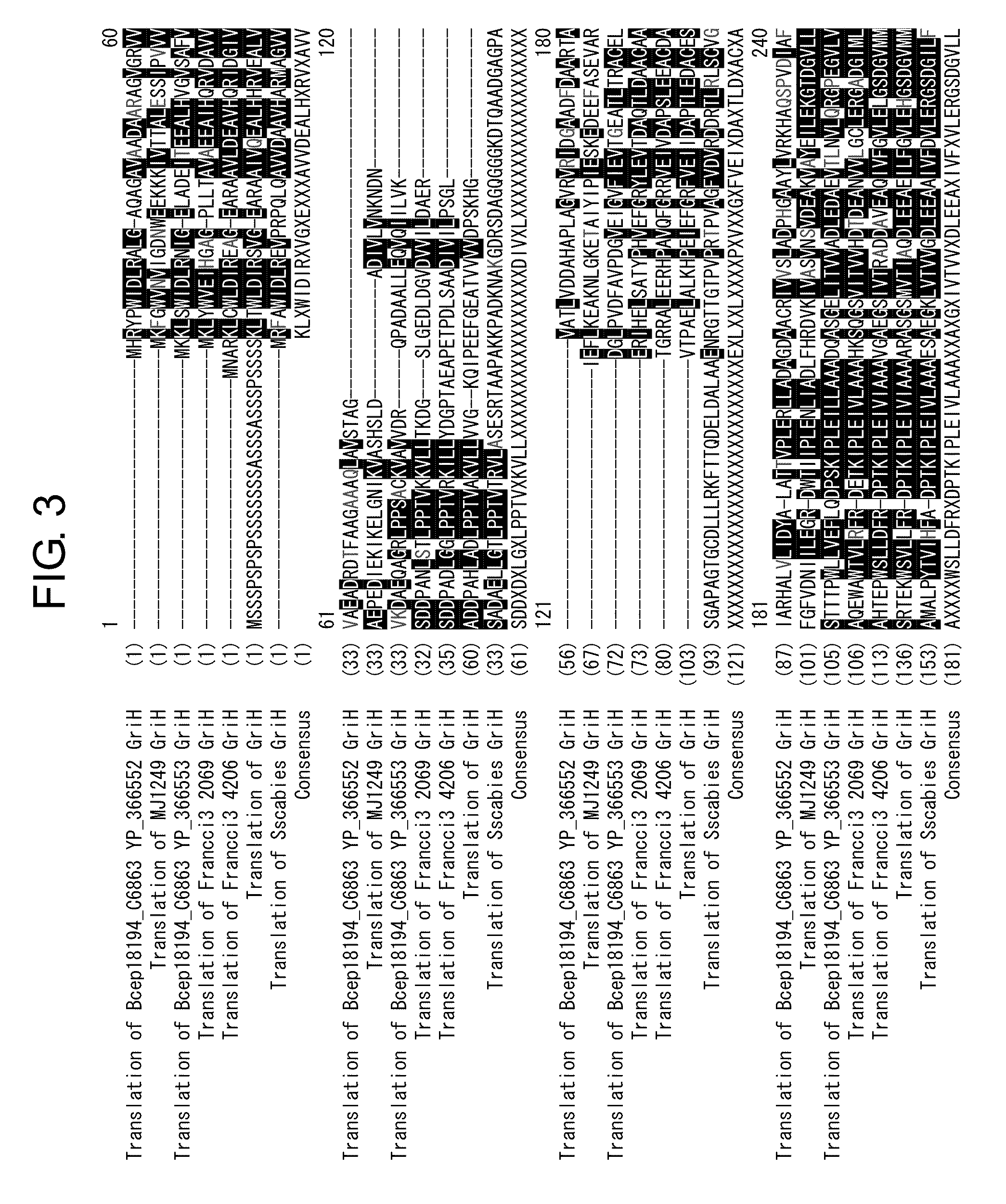
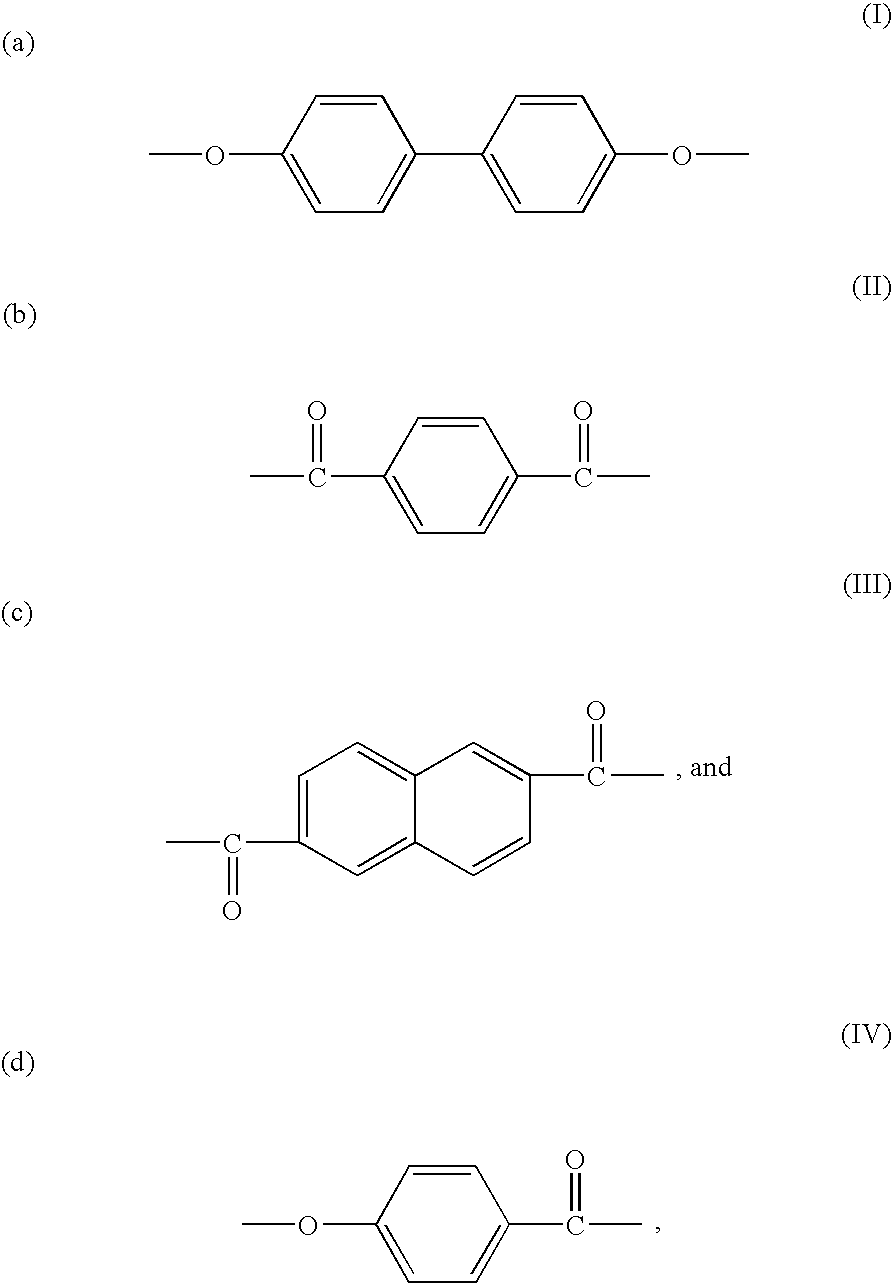
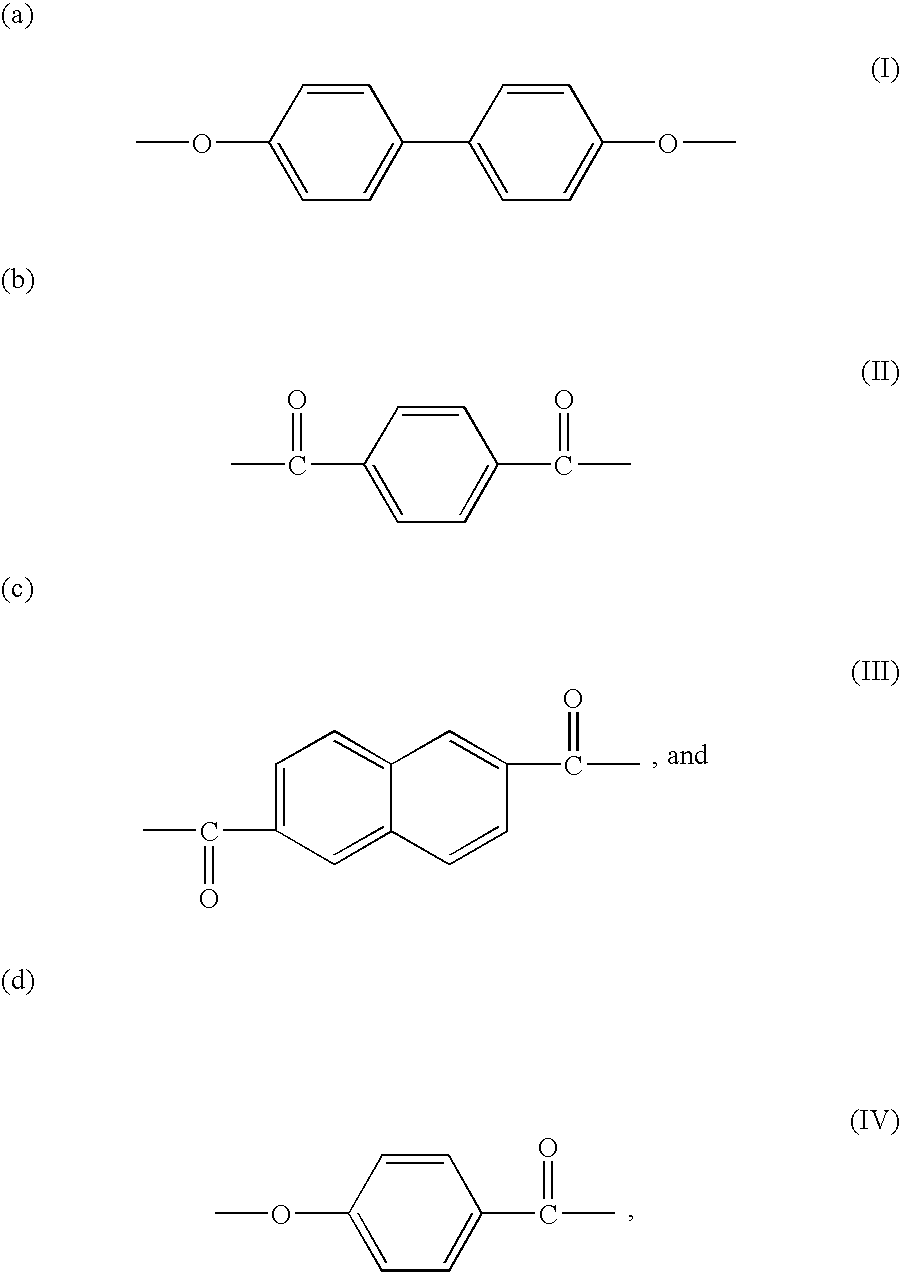
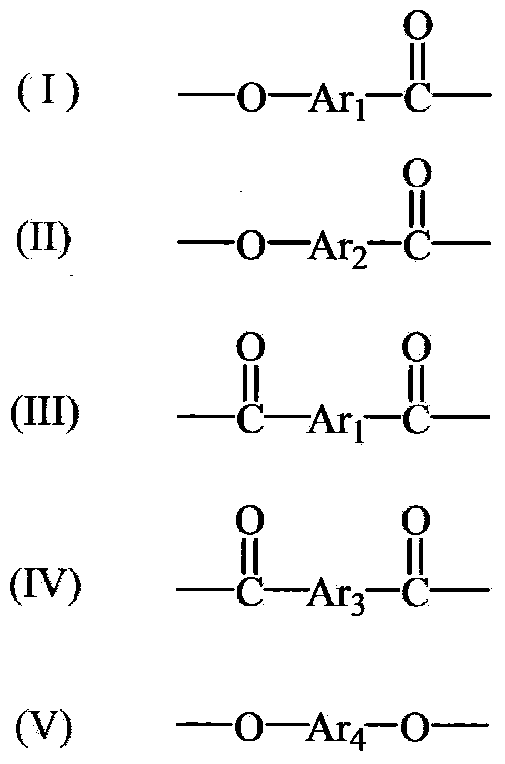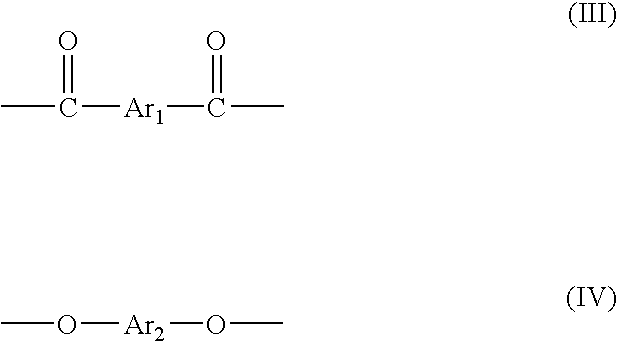Patents
Literature
107 results about "4-Hydroxybenzoic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
4-Hydroxybenzoic acid, also known as p-hydroxybenzoic acid (PHBA), is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar organic solvents such as alcohols and acetone. 4-Hydroxybenzoic acid is primarily known as the basis for the preparation of its esters, known as parabens, which are used as preservatives in cosmetics and some ophthalmic solutions. It is isomeric with 2-hydroxybenzoic acid, known as salicylic acid, a precursor to aspirin, and with 3-hydroxybenzoic acid.
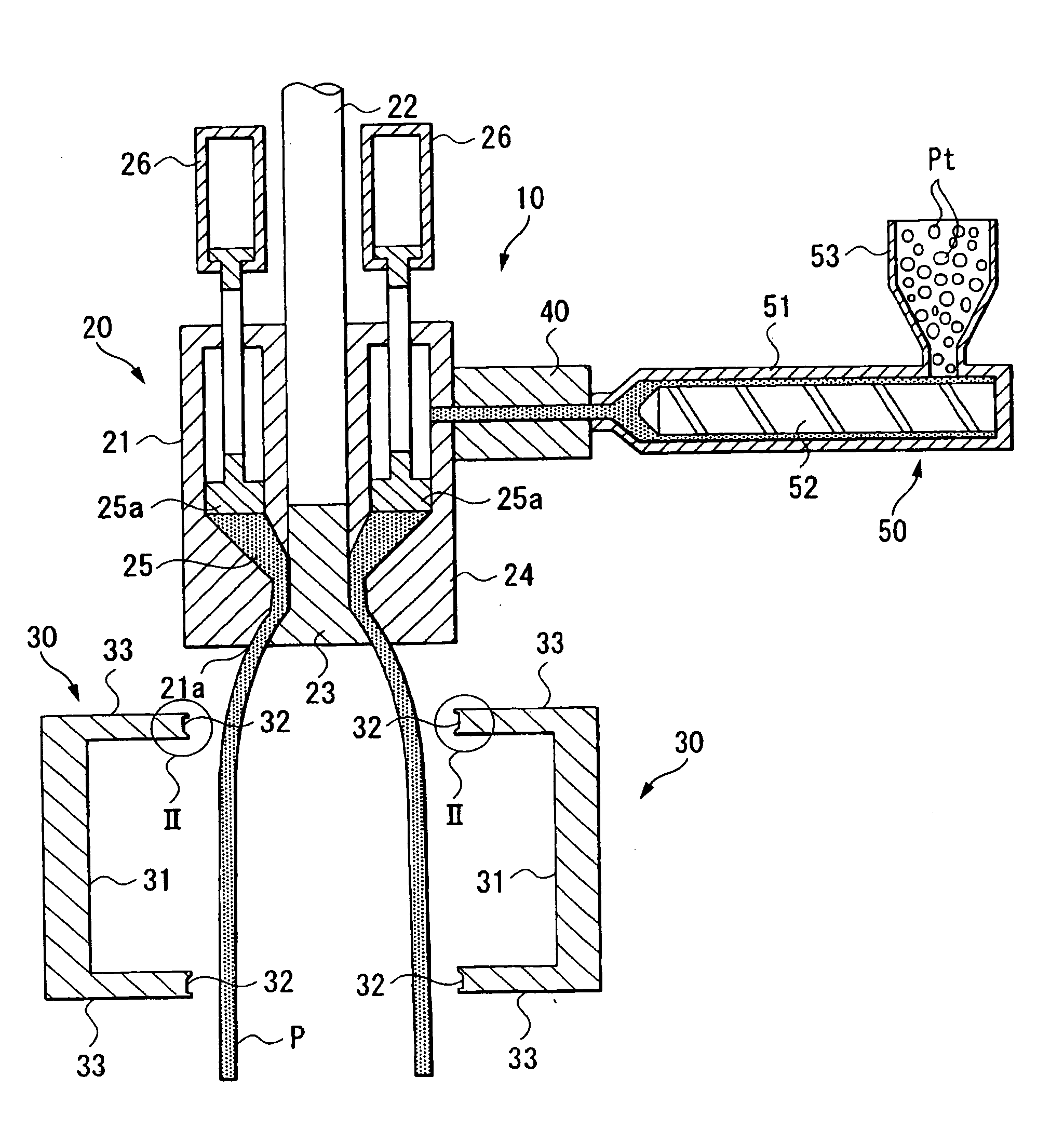
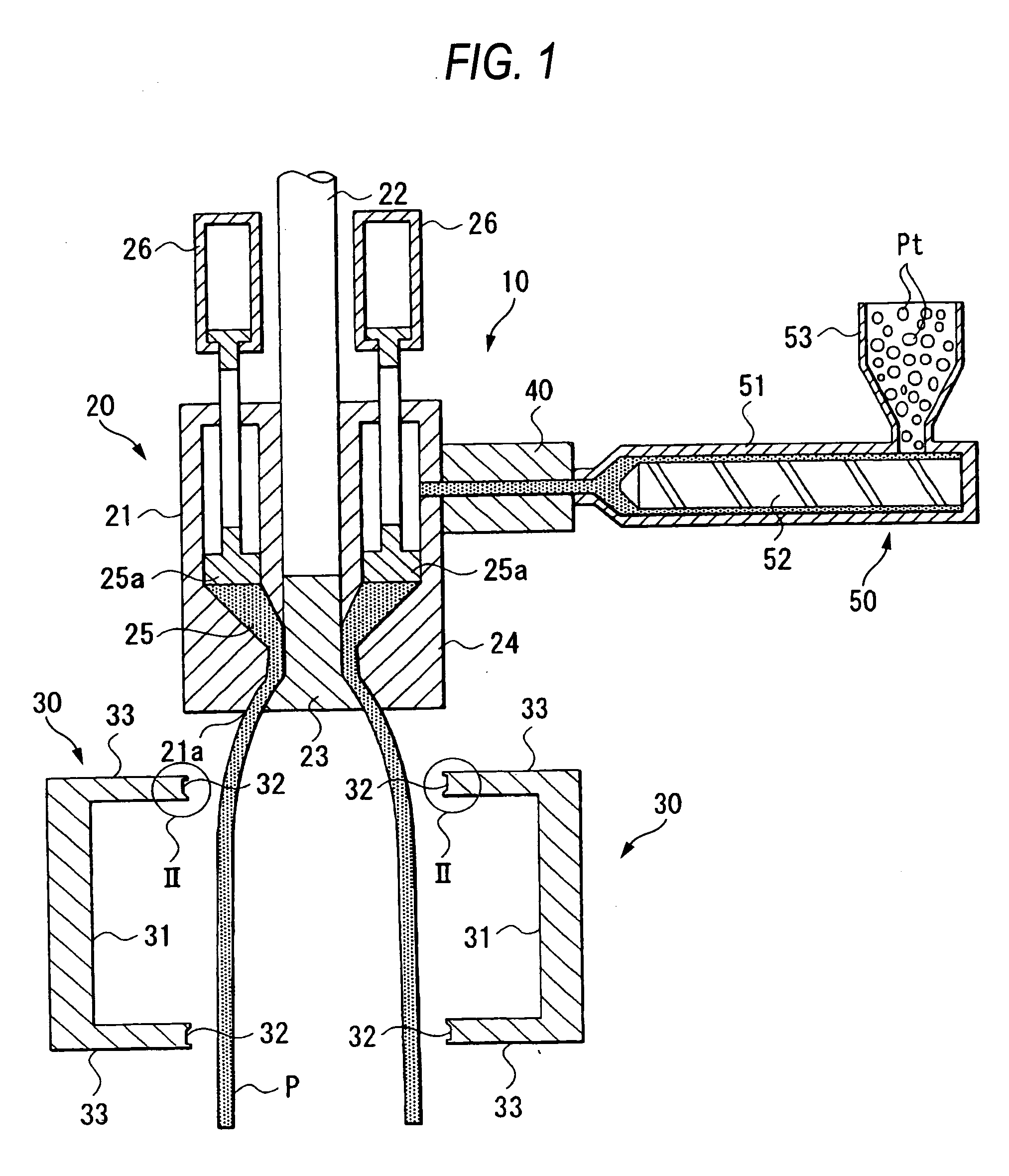
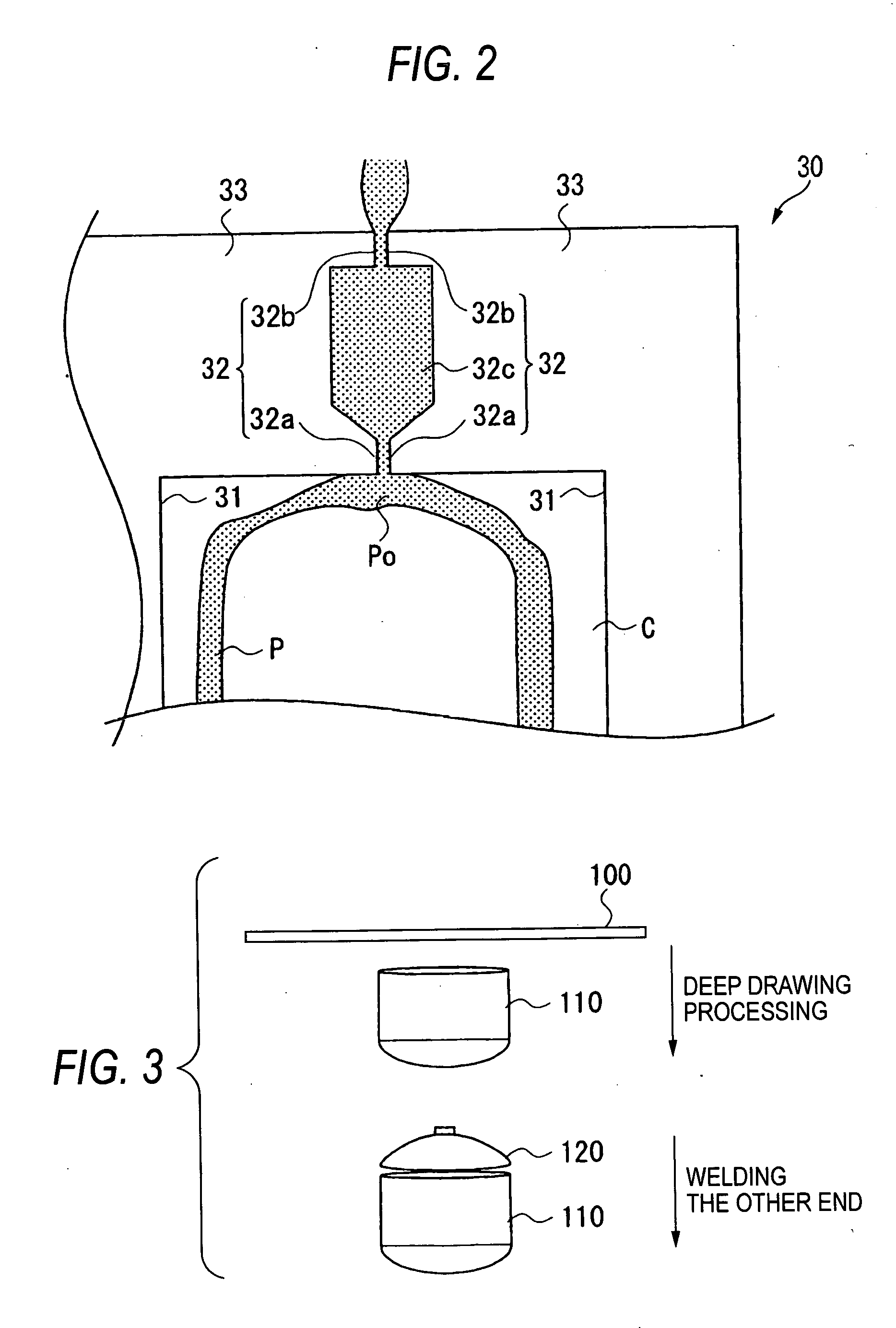
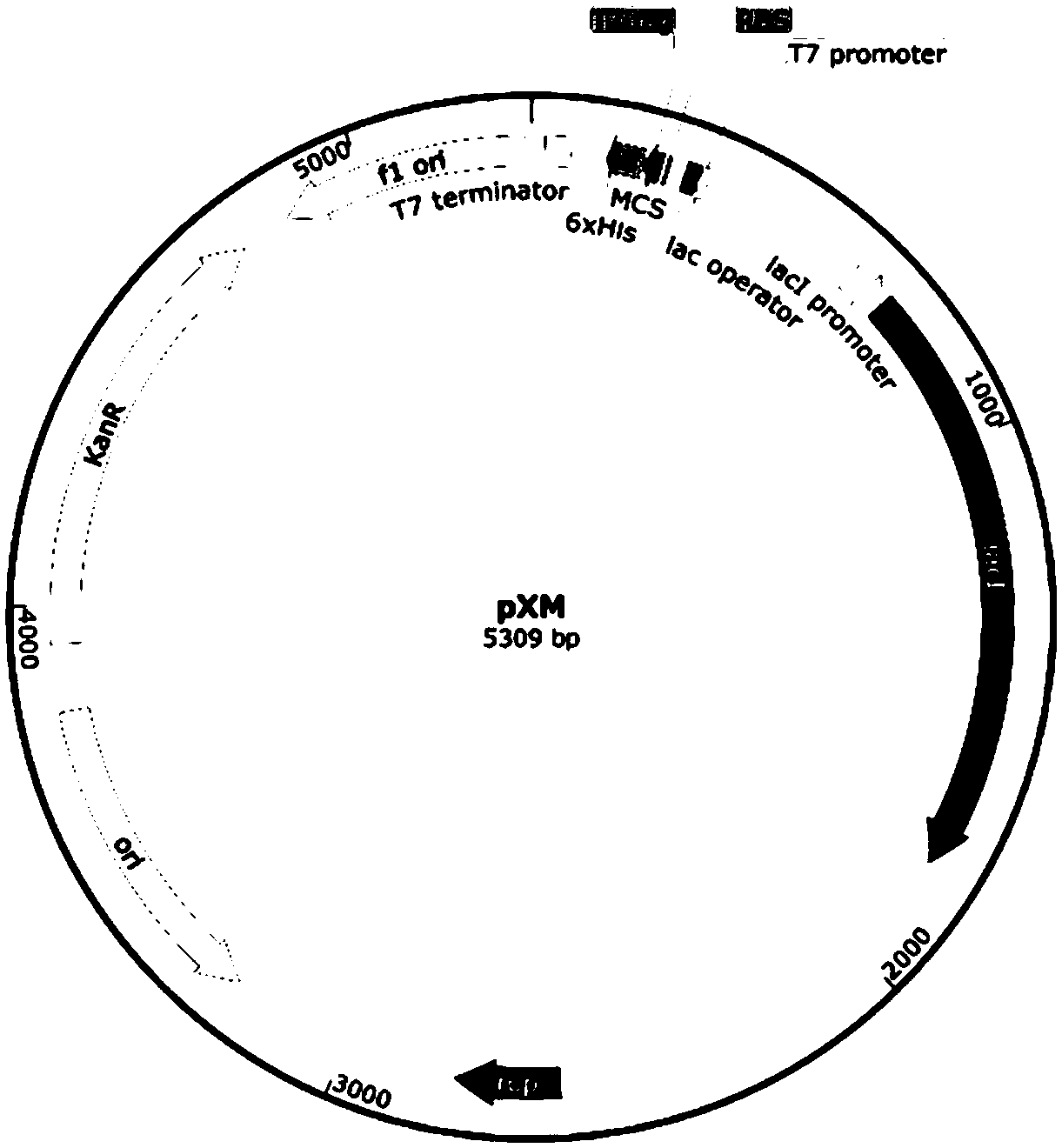
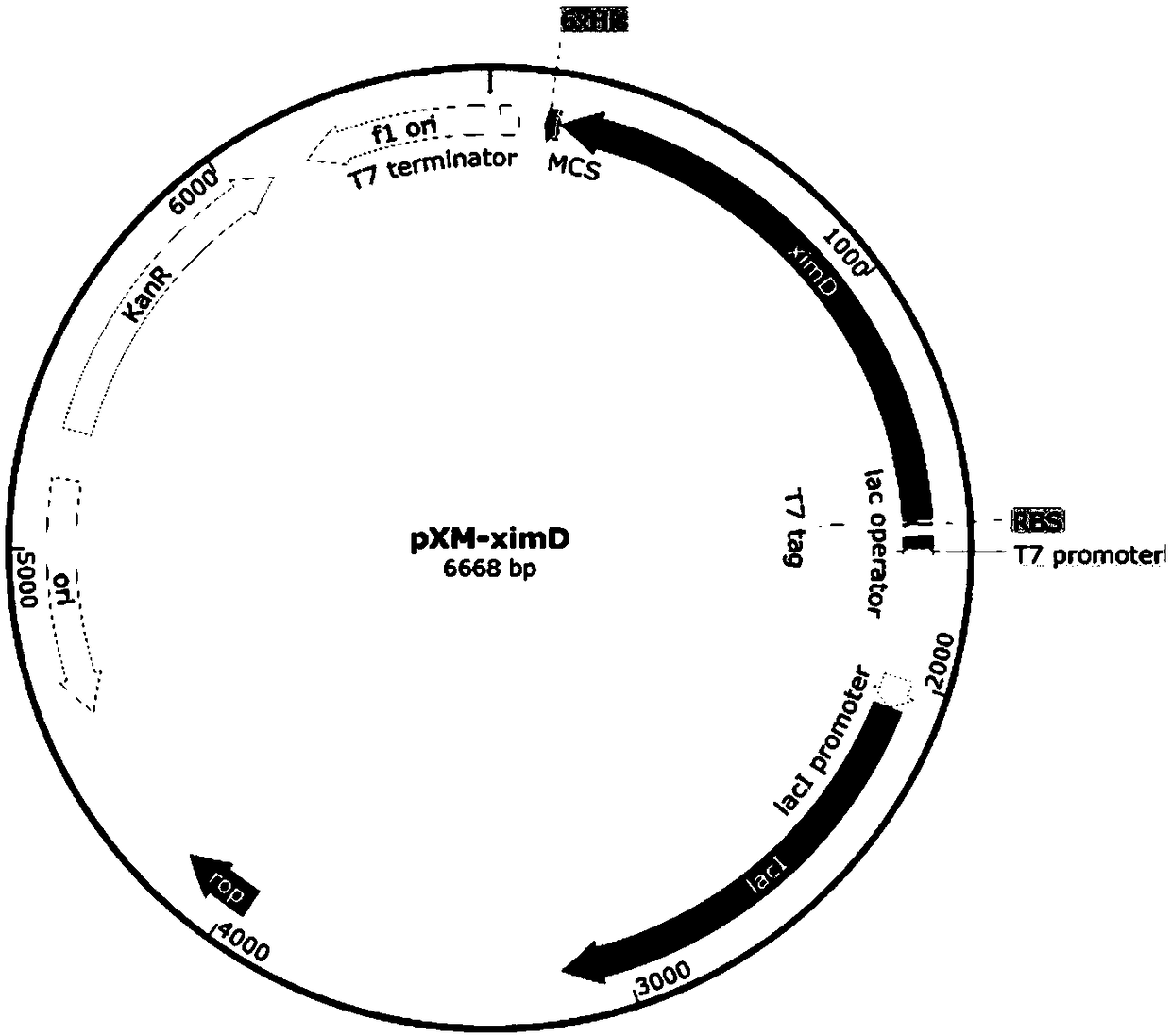
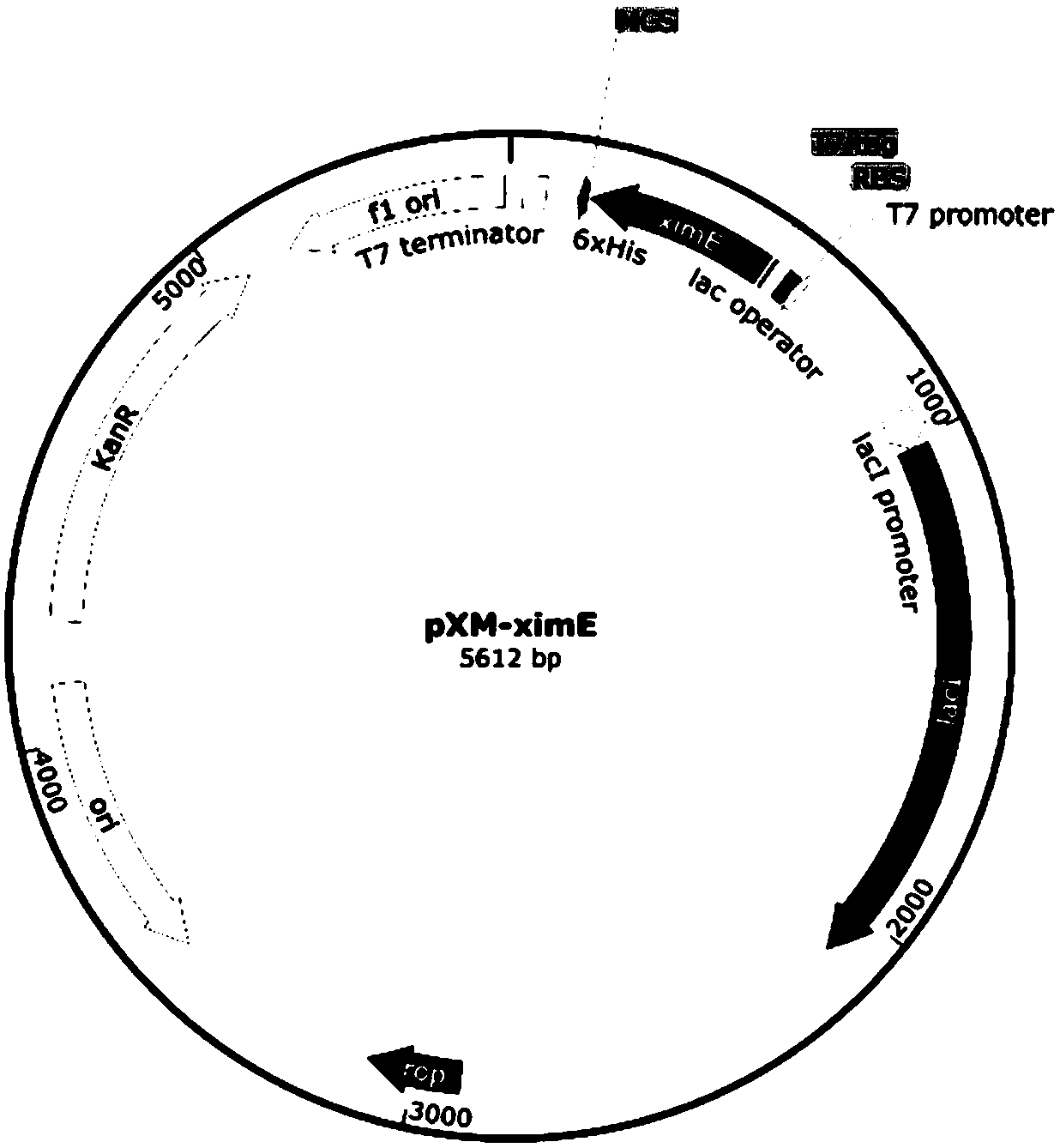
Method for manufacturing liner for pressure resistant container and liner made of liquid crystal resin
InactiveUS20050260372A1Favorable blow molding characteristicExcellent gas barrier propertiesLiquid crystal compositionsThin material handlingPolyesterPolymer science
A wholly aromatic polyester amide liquid crystal resin including repeating units of: (I) a 6-hydroxy-2-naphthoic acid residue: 1 to 15 mol %, (II) a 4-hydroxybenzoic acid residue: 40 to 70 mol %, (III) an aromatic diol residue: 5 to 28.5 mol %, (IV) a 4-aminophenol residue: 1 to 20 mol %, and (V) an aromatic dicarboxylic acid residue: 6 to 29.5 mol %, and having a melting point of 270° C. to 370° C., and having a melt viscosity of 60 Pa.s to 200 Pa.s at a shear rate of 1000 / sec at a temperature higher by 10° C. to 20° C. than this melting point is molten at a temperature of the melting point +40° C., and extruded at a rate of 0.3 kg / min or more and less than 5 kg / min to form a parison. A pair of molds arranged with the parison interposed there between are closed under a prescribed mold closing pressure, so that air is blown into the interior of the parison.
Owner:SUBARU CORP +1
Organic anti-reflective coating compositions for advanced microlithography
InactiveUS6846612B2Radiation applicationsSemiconductor/solid-state device manufacturingEpoxyAnti-reflective coating
New polymers and new anti-reflective compositions containing such polymers are provided. The compositions comprise a polymer (e.g., epoxy cresol novolac resins) bonded with a chromophore (4-hydroxybenzoic acid, trimellitic anhydride). The inventive compositions can be applied to substrates (e.g., silicon wafers) to form anti-reflective coating layers having high etch rates which minimize or prevent reflection during subsequent photoresist exposure and developing.
Owner:BREWER SCI
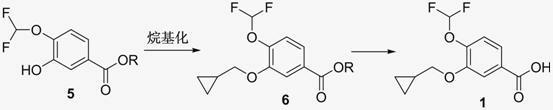
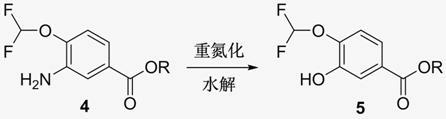
New method for synthesizing 3-cyclopropyl methoxy-4-(difluoromethoxy) benzoic acid
InactiveCN102093194AHigh purityHigh yieldPreparation from carboxylic acid esters/lactonesAlkyl transferBenzoic acid
The invention relates to a new method for synthesizing 3-cyclopropyl methoxy-4-(difluoromethoxy) benzoic acid. The method is characterized by taking 3-nitro-4-hydroxybenzoic acid ester as the raw material to obtain the 3-cyclopropyl methoxy-4-(difluoromethoxy) benzoic acid through alkylation, reduction, diazotization, hydrolysis, alkylation, deprotection and the like. The 3-cyclopropyl methoxy-4-(difluoromethoxy) benzoic acid is a key intermediate for synthesizing the drug roflumilast.
Owner:NANJING SIMCERE MEDICAL LAB CO LTD +1
Spontaneously color changing type thermal sensitive recording medium
A thermal sensitive recording medium which has a feature to change the color of recorded pattern slowly and spontaneously after development, and the recorded pattern which has several days passed can be easily distinguished from that of just after development. Said thermal sensitive recording medium contains 4-hydroxybenzoic acid ester represented by general formula (I) as a color developer and uses triphenylmethane-based leuco dye and reddish color developing leuco dye whose maximum absorption wave length is 450 DIFFERENCE 560 nm as a dye precursor. In this formula, R represents unsubstituted or substituted lower alkyl group of carbon number 1-7 or benzyl group.
Owner:NIPPON PAPER IND CO LTD
Liquid crystalline polyester resin composition
Owner:POLYPLASTICS CO LTD
Amorphous wholly aromatic polyester amide composition
InactiveUS20060073306A1Improve tensile propertiesImprove adhesionSynthetic resin layered productsThin material handlingPolyesterPolyolefin
The present invention is to provide an amorphous wholly aromatic polyester amide composition which has an excellent stretching property and a good adhesion to a heterogeneous polymer and thereby can be in particular suitably used for a multilayer film, or a multilayer sheet, a multilayer blow formed product and the like. That is, (the first invention) an amorphous wholly aromatic polyester amide composition obtained by blending 1 to 30% by weight of a modified polyolefin resin or a polyamide resin having a melting point of 230° C. or lower or being amorphous with an amorphous wholly aromatic polyester amide exhibiting an optical anisotropy at softening and flowing and being a wholly aromatic polyester amide obtained by copolymerizing (A) 4-hydroxybenzoic acid, (B) 2-hydroxy-6-naphthoic acid, (C) an aromatic aminophenol and (D) an aromatic dicarboxylic acid, wherein (1) the ratio of (C) the aromatic aminophenol is from 7 to 35% by mol, (2) the ratio of the bending monomer(s) among the starting monomers is from 7 to 35% by mol, (3) the ratio ((A) / (B)) between (A) 4-hydroxybenzoic acid and (B) 2-hydroxy-6-naphthoic acid is from 0.15 to 4.0, (4) the ratio of isophthalic acid is at least 35% by mol in (D) the aromatic dicarboxylic acid, (5) any melting point is not found by DSC measurement at a temperature rising rate of 20° C. / min and (6) the glass transition temperature is from 100 to 180° C., and (the second invention) an amorphous wholly aromatic polyester amide composition obtained by blending 1 to 30% by weight of a modified polyolefin resin or a polyamide resin having a melting point of 230° C. or lower or being amorphous with an amorphous wholly aromatic polyester amide exhibiting optical anisotropy at softening and flowing and being a wholly aromatic polyester amide obtained by copolymerizing (A) 4-hydroxybenzoic acid, (B) 2-hydroxy-6-naphthoic acid, (C)′ an aromatic diamine and (D) an aromatic dicarboxylic acid, wherein (1) the ratio of (C)′ the aromatic diamine is from 3 to 15% by mol, (2) the ratio of the bending monomer(s) is from 7 to 35% by mol in the starting monomers, (3) the ratio ((A) / (B)) between (A) 4-hydroxybenzoic acid and (B) 2-hydroxy-4-naphthoic acid is from 0.15 to 4.0, (4) any melting point is not found by DSC measurement at a temperature rising rate of 20° C. / min and (5) the glass transition temperature is from 100 to 180° C.
Owner:POLYPLASTICS CO LTD
Resin composition for extrusion molding and extrusion-molded article
InactiveUS7776410B2Specific melt viscosityLiquid crystal compositionsSynthetic resin layered productsPolyesterEpoxy
A purpose of the present invention is to obtain a hollow extruded article by easy blow molding or extrusion molding to give a draw down resistance and a uniform wall thickness of the article without damaging low gas permeability which is a characteristic property of a liquid-crystalline polymer. A resin composition for extrusion molding which is prepared by melting and kneading 99-70 wt. % of wholly aromatic polyester amide liquid crystal resin (A) having a melting point of 270-370° C. and a melt viscosity at 1000 / sec. shear rate of 20-60 Pa·s at Temperature T1 which is higher than 20° C. from said melting point, and containing (I) 1-15 mole % of 6-hydroxy-2-naphthoic acid residue, (II) 40-70 mole % of 4-hydroxybenzoic acid residue, (III) 5-28.5 mole % of aromatic diol residue, (IV) 1-20 mole % of 4-aminophenol residue, and (V) 6-29.5 mole % of aromatic dicarboxylic acid residue; and 1-30 wt. % of epoxy modified polyolefin type resin (B), and has a melt viscosity at 1000 / sec. shear rate of 60-4000 Pa·s at said Temperature T1, and a melt tensile strength of 20 mN or more at 14.8 m / min. draw rate is used.
Owner:POLYPLASTICS CO LTD
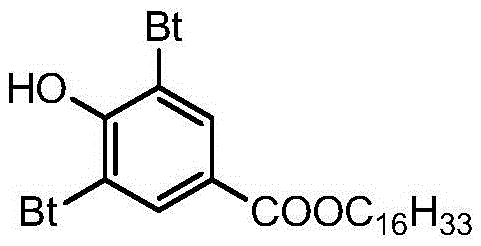
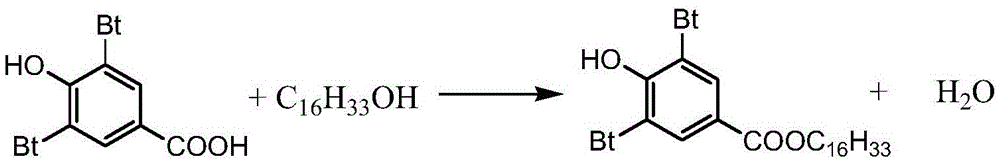
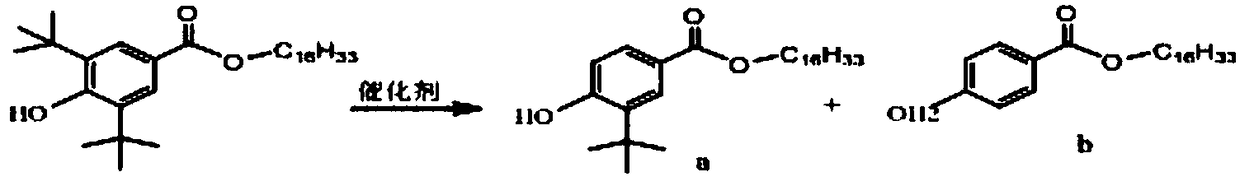
Preparing method for 3,5-bis(tertiary butyl)-4-hydroxybenzoic acid hexadecane alkyl ester
InactiveCN105541613ASave consumptionSave energyOrganic compound preparationCarboxylic acid esters preparationHexadecaneOrganic solvent
The invention provides a preparing method for 3,5-bis(tertiary butyl)-4-hydroxybenzoic acid hexadecane alkyl ester. The preparing method comprises the steps that 3,5-bis(tertiary butyl)-4-hydroxybenzoic acid, cetyl alcohol and a catalyst are added into a reaction vessel, flow of inert gas is introduced, a heat-preserving reaction is carried out at the temperature of 80 DEG C-160 DEG C, TLC tracks and monitors the reaction till the reaction endpoint, and reaction liquid is subjected to after-treatment to obtain 3,5-bis(tertiary butyl)-4-hydroxybenzoic acid hexadecane alkyl ester. By means of the preparing method, it is unnecessary to adopt other solvent, production cost is saved, and waste gas exhaustion of organic solvent is avoided in the synthesis process; the inert gas serves as a water-carrying agent and a protective agent, operation and control are simple, production safety is good, the color of products is improved, and the method has remarkable economic benefits and has remarkable environmental protection significance.
Owner:ZHEJIANG NORMAL UNIVERSITY
Vapor phase corrosion inhibitors and method for their production
InactiveUS7824482B2Large scaleEasy to processOther chemical processesSynthetic resin layered productsAlloyHumid climate
The present invention relates to new substance combinations as vapor phase corrosion inhibitors for protecting a broad range of customary utility metals, including iron, chromium, nickel, tin, zinc, aluminum, copper, magnesium and their alloys, against corrosion in humid climates. Said substance combinations comprise (1) at least one C6 to C10 aliphatic monocarboxylic acid, (2) at least one C6 to C10 aliphatic dicarboxylic acid, and (3) a primary aromatic amide. Preferably they further comprise (4) an aliphatic ester of hydroxybenzoic acid, in particular of 4-hydroxybenzoic acid, and / or (5) a bemzimidazole, in particular a benzimidazole substituted on the benzene ring.
Owner:EXCOR KORROSIONSFORSCHUNG
Vapor phase corrosion inhibitors and method for their production
InactiveCN101457363AEasy and hazard-free processingLow costOther chemical processesSynthetic resin layered productsAlloyHumid climate
The present invention relates to new substance combinations as vapor phase corrosion inhibitors for protecting a broad range of customary utility metals, including iron, chromium, nickel, tin, zinc, aluminum, copper, magnesium and their alloys, against corrosion in humid climates. Said substance combinations comprise (1) at least one C6 to C10 aliphatic monocarboxylic acid, (2) at least one C6 to C10 aliphatic dicarboxylic acid, and (3) a primary aromatic amide. Preferably they further comprise (4) an aliphatic ester of hydroxybenzoic acid, in particular of 4-hydroxybenzoic acid, and / or (5) a bemzimidazole, in particular a benzimidazole substituted on the benzene ring.
Owner:EXCOR KORROSIONSFORSCHUNG
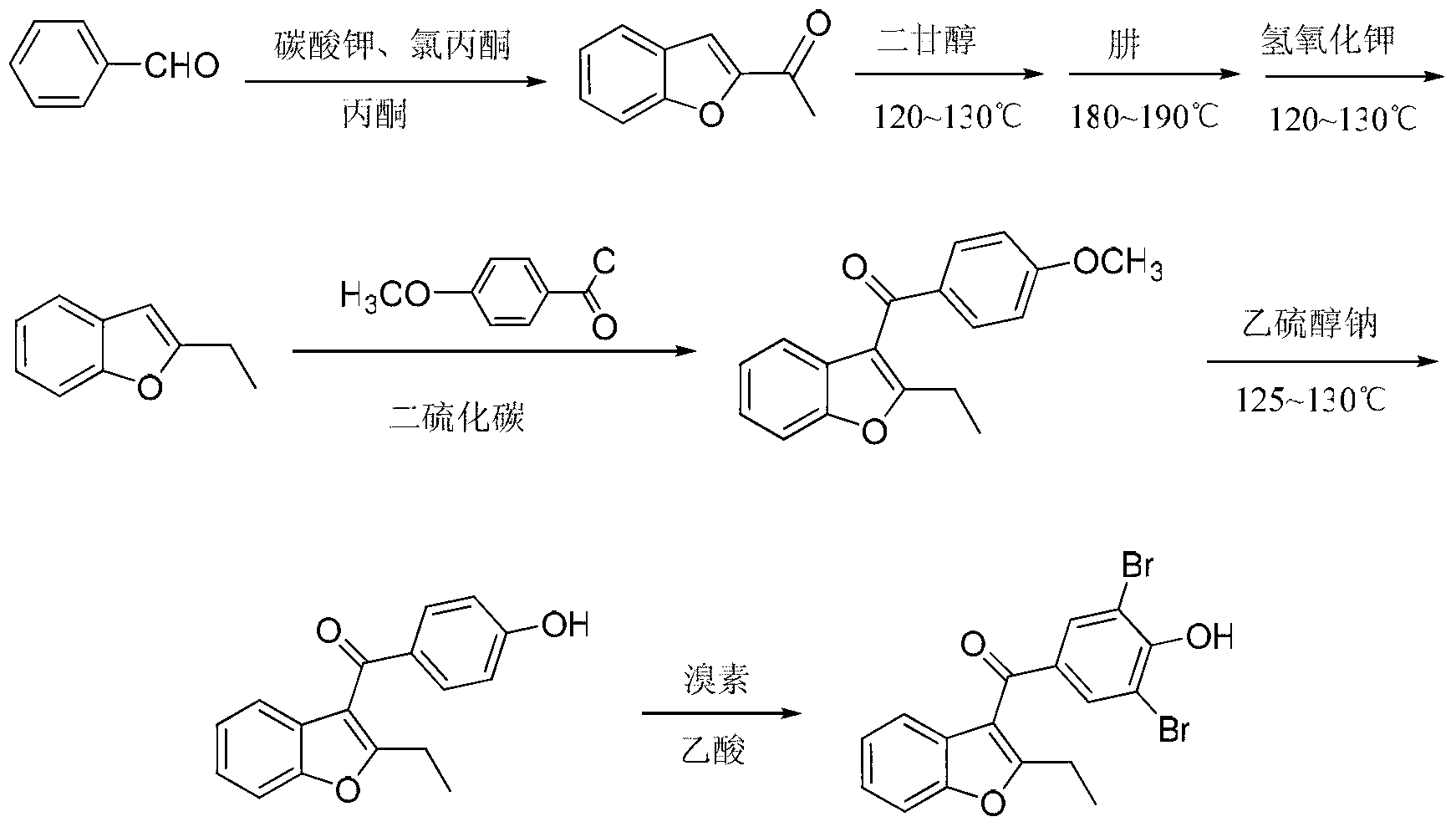
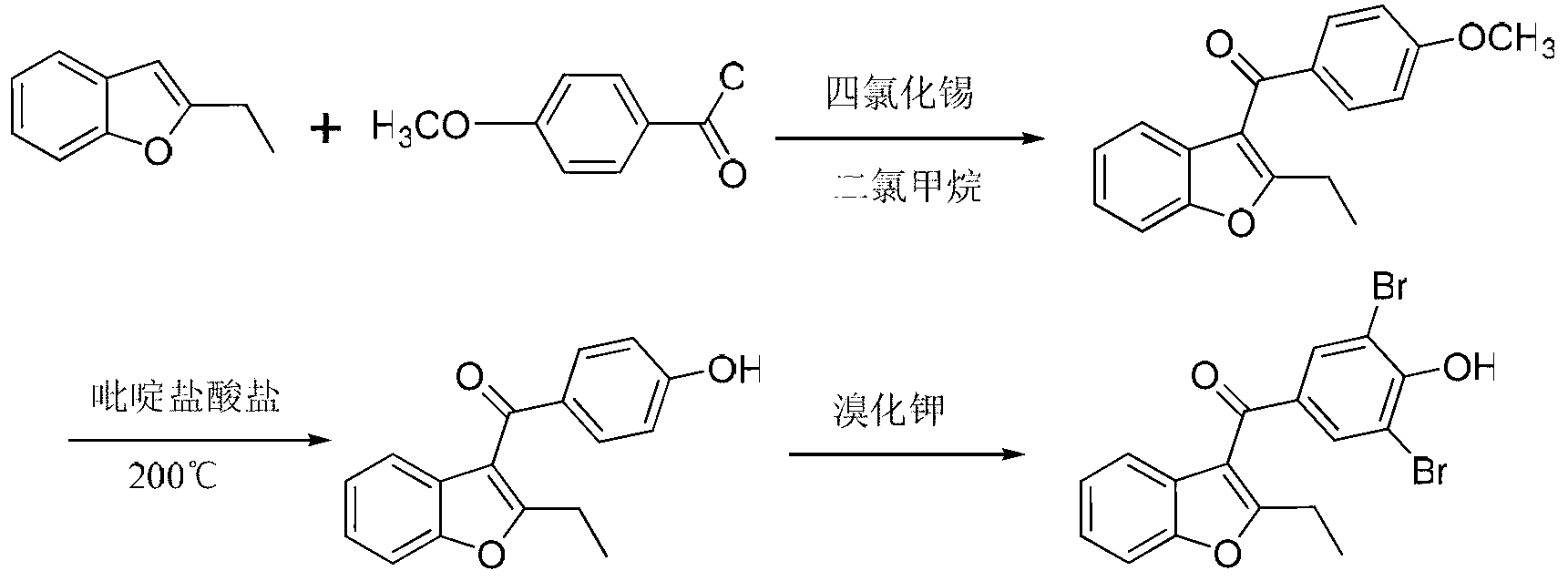
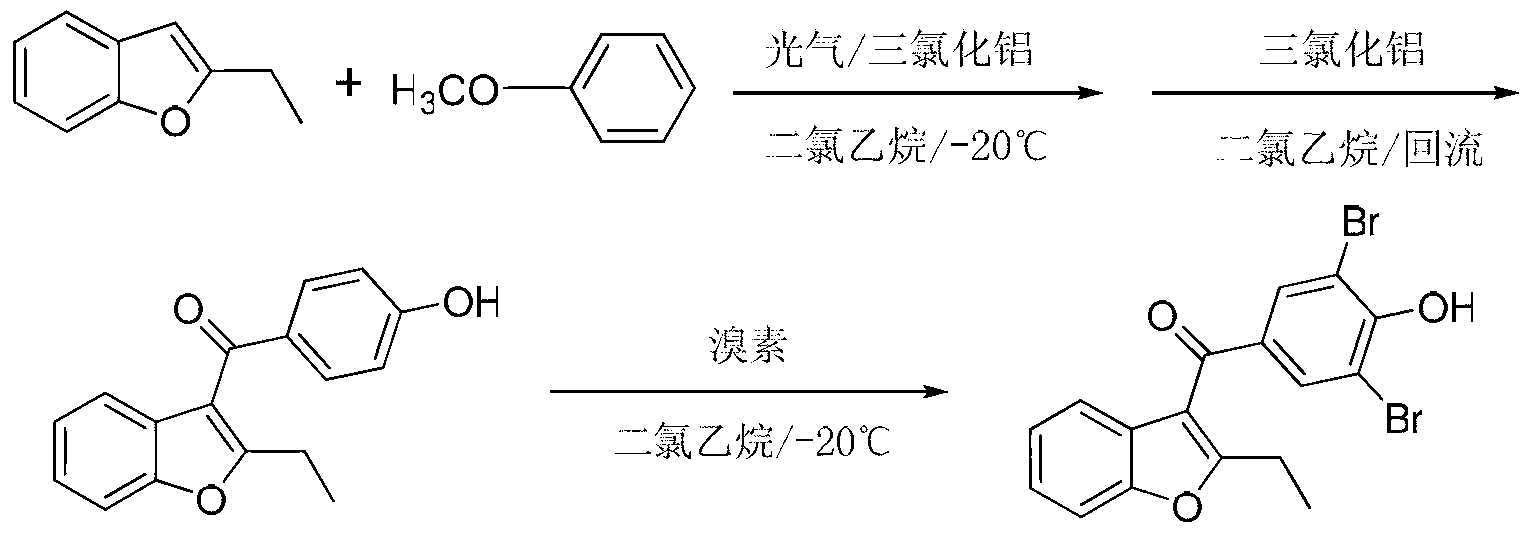
Method for preparing benzbromarone
The invention relates to the field of drug synthesis and in particular relates to a method for preparing benzbromarone. The method for preparing benzbromarone is characterized by comprising the steps of carrying out an acetylation reaction, a Friedel-Craft reaction, a hydrolysis reaction and the like to prepare the benzbromarone by using 3,5-dibromo-4-hydroxybenzoic acid and 2-ethyl benzofuran as raw materials. The preparation method disclosed by the invention has the advantages of being simple and safe to operate, convenient for post-treatment, high in product purity, low in economic cost, small in environment pollution, easier to realize industrialization and the like.
Owner:HEFEI IND PHARMA INST
Fully aromatic polyester and polyester resin composition
InactiveCN103459459AImprove heat resistanceImprove toughnessMonocomponent copolyesters artificial filamentThin material handlingStructural unitPolymerization
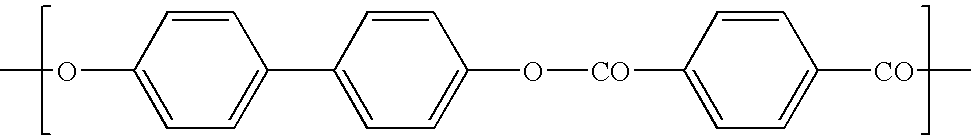
The present invention provides a fully aromatic polyester that can be produced in a normal polymerization device and that has superior heat resistance and ductility. The fully aromatic polyester comprises: a constituent unit (I) derived from 4-hydroxybenzoic acid; a constituent unit (II) derived from 6-hydroxy-2-naphthoic acid; a constituent unit (III) derived from 1,4-phenylene dicarboxylic acid; a constituent unit (IV) derived from 1,3-phenylene dicarboxylic acid; and a constituent unit (V) derived from 4,4'-dihydroxybiphenyl. The fully aromatic polyester exhibits optical anisotropy when melted and, with respect to all the constituent units, there are 35-75 mol% of constituent unit (I), 2-8 mol% of constituent unit (II), 4.5-30.5 mol% of constituent unit (III), 2-8 mol% of constituent unit (IV), 12.5-32.5 mol% of constituent unit (V), and 4-10 mol% of constituent units (II)+(IV).
Owner:POLYPLASTICS CO LTD
Liquid crystalline polymer composition
PCT No. PCT / US97 / 00068 Sec. 371 Date Jun. 25, 1998 Sec. 102(e) Date Jun. 25, 1998 PCT Filed Jan. 2, 1997 PCT Pub. No. WO97 / 25363 PCT Pub. Date Jul. 17, 1997A thermotropic liquid crystalline polymer constituted of repeat units derived from hydroquinone, terephthalic acid, isophthalic acid, 2,6-naphthalene dicarboxylic acid, and 4-hydroxybenzoic acid, all present in defined amounts. Such polymer has excellent physical properties and high temperature stability. It is suitable as a molding resin.
Owner:TICONA LLC
Liquid crystal polymers
InactiveUS7148311B2Good physical and mechanical propertiesLower melting temperatureLiquid crystal compositionsOrganic chemistryFlexural strengthThermal expansion
Owner:RENESSELAER POLYTECHNIC INST
Liquid-crystal polymer and molded articles
InactiveUS20120190813A1Improve thermal conductivityHigh mechanical strengthLiquid crystal compositionsLiquid crystallineN-Acetyl-p-aminophenol
To provide an easily-manufactured liquid-crystalline polymer having high thermal conductivity, as well as a molded article that is formed of the liquid crystalline polymer and has a property of being highly mechanical. A liquid-crystalline polymer is used that is formed by polymerizing monomers having an asymmetrical molecular structure, in which the enthalpy of fusion ΔH measured by way of DSC (Differential Scanning calorimetry) is greater than or equal to 2.5 J / g (joules per gram) and less than or equal to 10 J / g, and the inherent viscosity (I.V.) is greater than or equal to 5 dl / g and less than or equal to 7 dl / g. It is preferable for the monomer having asymmetrical molecular structure to be at least one selected from a group consisting of 4-hydroxybenzoic acid (HBA), 6-hydroxy-2-naphthoic acid (HNA), N-acetyl-p-aminophenol (APAP), and 4-hydroxy-4′-biphenylcarboxylic acid (HBCA).
Owner:POLYPLASTICS CO LTD
Method for manufacturing thermotropic liquid-crystalline polymer
InactiveUS7087704B2Not harm and low effectLiquid crystal compositionsImpression capsLiquid crystallineHydroxybenzoic acid
It is an object of the present invention to manufacture high quality thermotropic liquid-crystalline polymer generating no low boiling gas and inducing no discoloration resulted from thermal degradation at high yield. To manufacture a liquid-crystalline polymer having 50 mol % or larger ratio of constitutive unit introduced from 4-hydroxybenzoic acid, the reaction is conducted under the presence of an acylating agent and of a catalyst quantity of aromatic sulfonic acid.
Owner:POLYPLASTICS CO LTD
Method for producing an aminohydroxybenzoic acid-type compound
Provided is a method for efficiently producing a 3-amino-4-hydroxybenzoic acid-type compound by culturing a coryneform bacterium that has a gene encoding a mutated aspartokinase not subject to feedback inhibition, and that is transformed with a recombinant vector containing a DNA encoding a protein having an activity to form 3-amino-4-hydroxybenzoic acid from dihydroxyacetone phosphate and aspartate semialdehyde.
Owner:AJINOMOTO CO INC
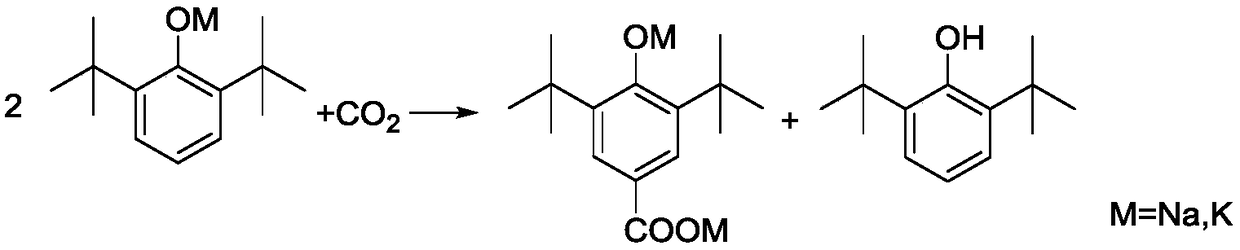
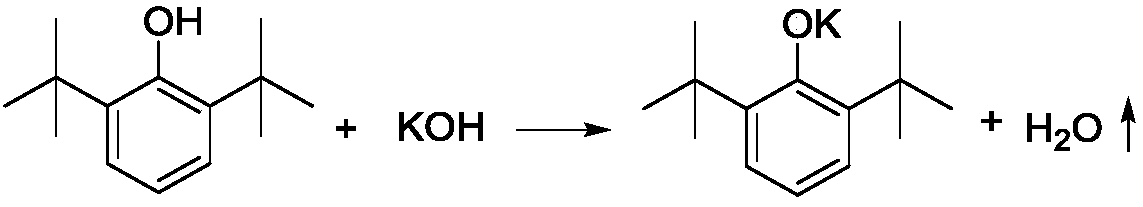
Production method of 3,5-di-tert-butyl-4-hydroxybenzoic acid
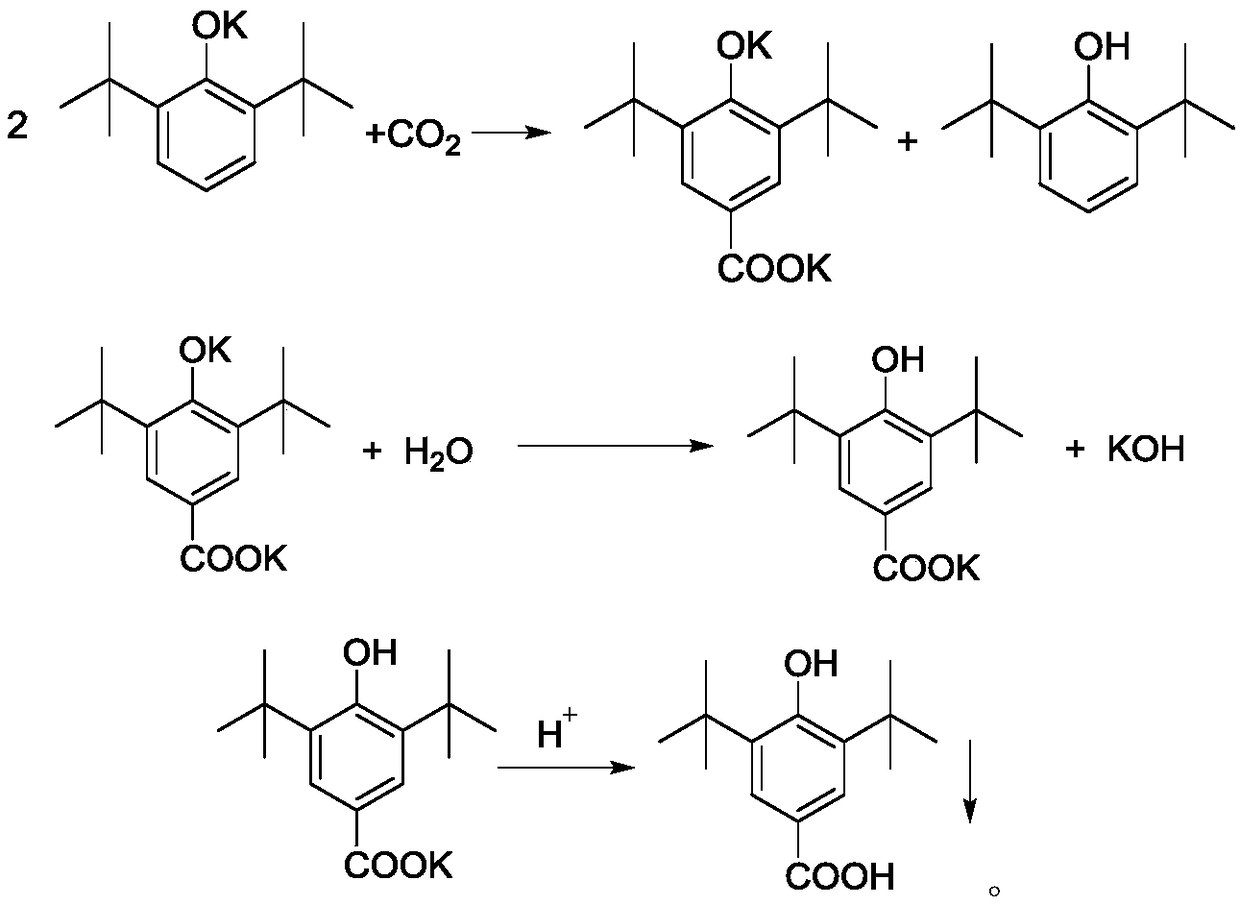
ActiveCN109096099APrevent spontaneous combustionAvoid problemsPreparation from carboxylic acid saltsOrganic compound preparationKolbe–Schmitt reactionSchmidt reaction
The invention discloses a production method of 3,5-di-tert-butyl-4-hydroxybenzoic acid. The production method sequentially comprises the steps as follows: 2,6-di-tert-butylphenol, a potassium hydroxide aqueous solution and toluene are added to a reactor under protection of nitrogen to be subjected to a reflux reaction under a water distribution condition; CO2 is introduced into the reactor until the pressure is 0.1-3.0 MPa, a Kolbe-Schmitt reaction is conducted under a pressure holding condition, after the reaction, corresponding aftertreatment is carried out, and a crude product of 3,5-di-tert-butyl-4-hydroxybenzoic acid is obtained. The method has short reaction time and high conversion rate, has obvious advantages as compared with traditional gas-solid processes and existing solvent-process synthesis processes and is suitable for further industrialization.
Owner:安徽新秀化学股份有限公司 +1
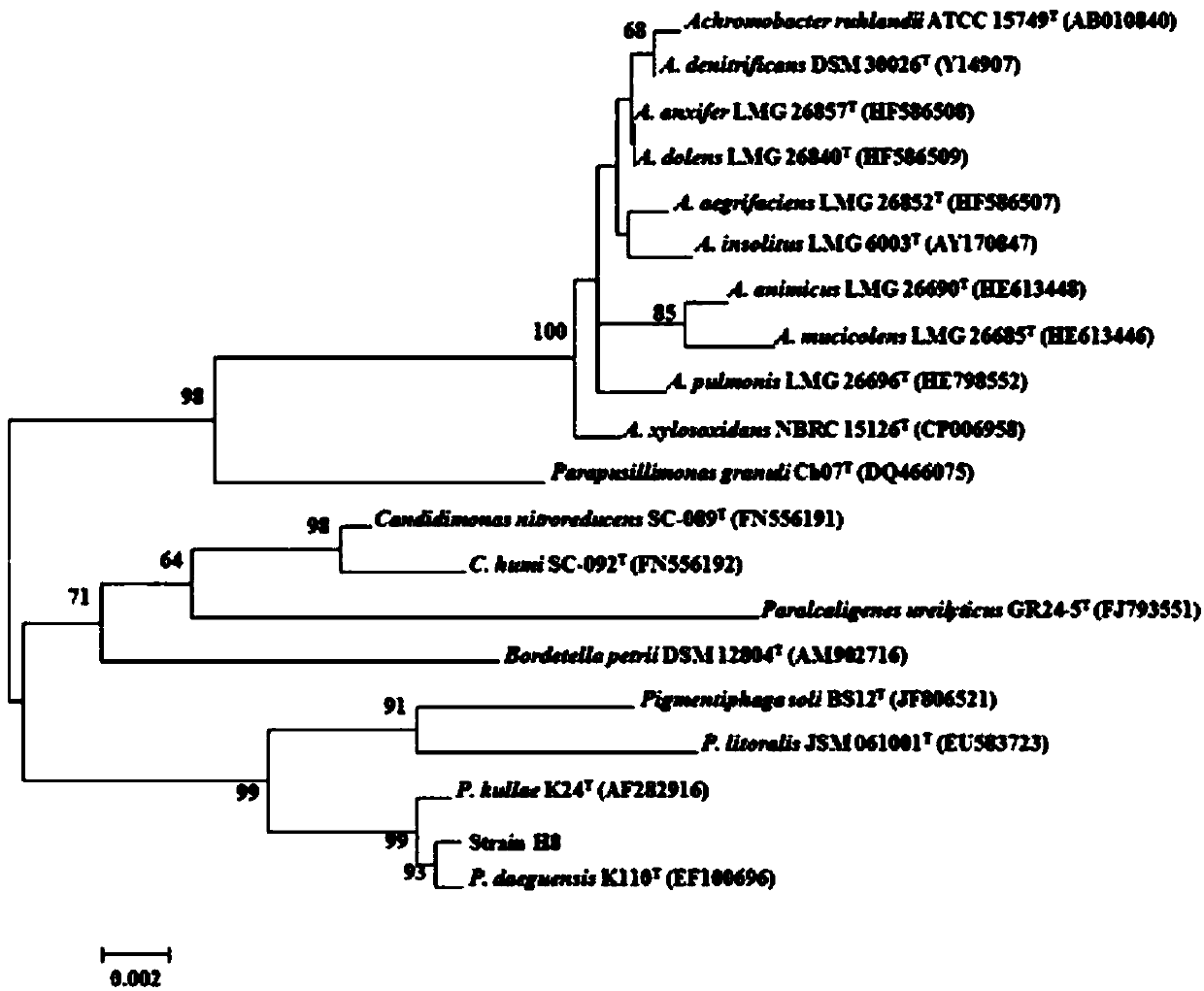
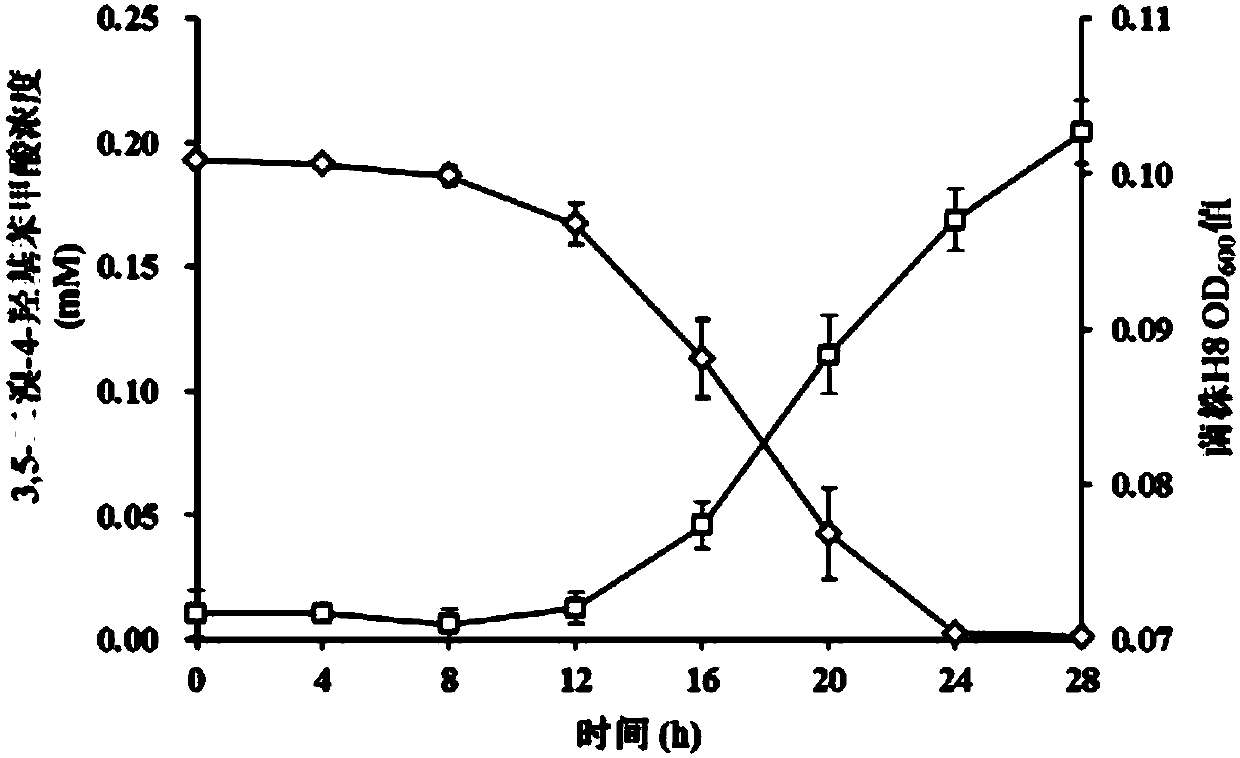
Halogenated benzoic acid degradation strain, inoculant produced by strain and application
InactiveCN107674847AImprove degradation efficiencyBroad degradation spectrumBacteriaWater contaminantsBenzoic acidAromatic hydrocarbon
The invention discloses a halogenated benzoic acid degradation strain, an inoculant produced by the strain and an application. The halogenated benzoic acid degradation strain H8 is preserved in ChinaCenter for Type Culture Collection on April 28, 2017 with preservation number of CCTCC NO.M2017222. The invention provides the halogenated benzoic acid degradation inoculant which is produced by the halogenated benzoic acid degradation strain H8. The bacterium H8 provided by the invention can be used for effectively and rapidly degrading halogenated benzoic acid. The degradation strain H8 has a relatively wide degradation spectrum and is capable of degrading such halogenated aromatic hydrocarbon as 3,5-dibromo-4-hydroxybenzoic acid, 3-bromo-4-hydroxybenzoic acid, 3,5-dichloro-4-hydroxybenzoicacid, 3-chloro-4-hydroxybenzoic acid and the like. The halogenated benzoic acid degradation strain can be used for conditioning halogenated benzoic acid pollution.
Owner:NANJING AGRICULTURAL UNIVERSITY
Liquid crystal high molecular material and its preparing method
InactiveCN1492023AEasy to manufactureEasy to useLiquid crystal compositionsLiquid crystalP-hydroxybenzoic acid
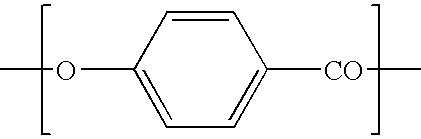
The liquid crystal polymer (LCP) material contains in its chain structure repeated units introducing by p-hydroxybenzoic acid, hydroquinol, mixed aromatic acid, terephthalic acid and 4, 4'-diphenyl diformic acid. Adding small amount of 4, 4'-diphenyl diformic acid in certain range can lower the smelting point of LCP to expand its processing temperature window; lower the rate and melt viscosity of its late sage polymerization for easy control; and raise its toughness. In some composition range, the processing temperature window has its width increased with the increasing molar content of p-hydroxybenzoic acid and terephthalic acid.
Owner:SHANGHAI PRET COMPOSITES +1
Method for producing an aminohydroxybenzoic acid-type compound
InactiveUS8093346B2High expressionHydrolasesOxidoreductasesDihydroxyacetone phosphateHydroxybenzoic acid
Provided is a method for efficiently producing a 3-amino-4-hydroxybenzoic acid-type compound by culturing a coryneform bacterium that has a gene encoding a mutated aspartokinase not subject to feedback inhibition, and that is transformed with a recombinant vector containing a DNA encoding a protein having an activity to form 3-amino-4-hydroxybenzoic acid from dihydroxyacetone phosphate and aspartate semialdehyde.
Owner:AJINOMOTO CO INC
Liquid crystalline polymer composition
ActiveUS20040140450A1Liquid crystal compositionsSynthetic resin layered productsLiquid crystallinePolymer science
Liquid crystalline polymer having repeat units derived from 4,4'-biphenol, terephthalic acid, 2,6-naphthalenedicarboxylic acid, and 4-hydroxybenzoic acid in a limited compositional range have melting points of 400° C. or more, and are useful for molded articles and for films, particularly for uses where good high temperature resistance is needed.
Owner:TICONA LLC
High-temperature-resistant polyester lubricating oil base oil and preparation method thereof
ActiveCN109233936AGuaranteed high temperature performanceModerate viscosity indexBase-materialsPerfluoroheptanoic acidPolyester
The invention discloses high-temperature-resistant polyester lubricating oil base oil and a preparation method thereof, and belongs to the field of fine chemical engineering. The base oil is preparedfrom 1,3,5-cyclohexane trimethyl alcohol, diethyl fluoromalonate, 12-benzoyl stearic acid, 2,2-difluoropropionic acid, 3-fluoro-4-hydroxybenzoic acid, perfluoroheptanoic acid and tris(trimethylsilyl)phosphate; fluorine-contained carboxylic acid and the 1,3,5-cyclohexane trimethyl alcohol with high heat resistance are used as main raw materials, the 12-benzoyl stearic acid and the 3-fluoro-4-hydroxybenzoic acid are introduced to adjust the viscosity index of the base oil, and the tris(trimethylsilyl) phosphate is used as a high-temperature-resistant antioxidant; through fluorinated compounds in the raw materials, the high temperature resistance and the carbon deposition resistance of the product are guaranteed. The preparation method has the advantages that the raw materials are easy to obtain, and the method is simple and convenient to operate; the prepared lubricating oil base oil has good high-temperature resistance, a moderate viscosity index, good lubricating performance and wearresistance, can be applied to lubrication of most automotive engines, and is especially applicable to high temperature environments.
Owner:上海新纳克合成材料有限公司
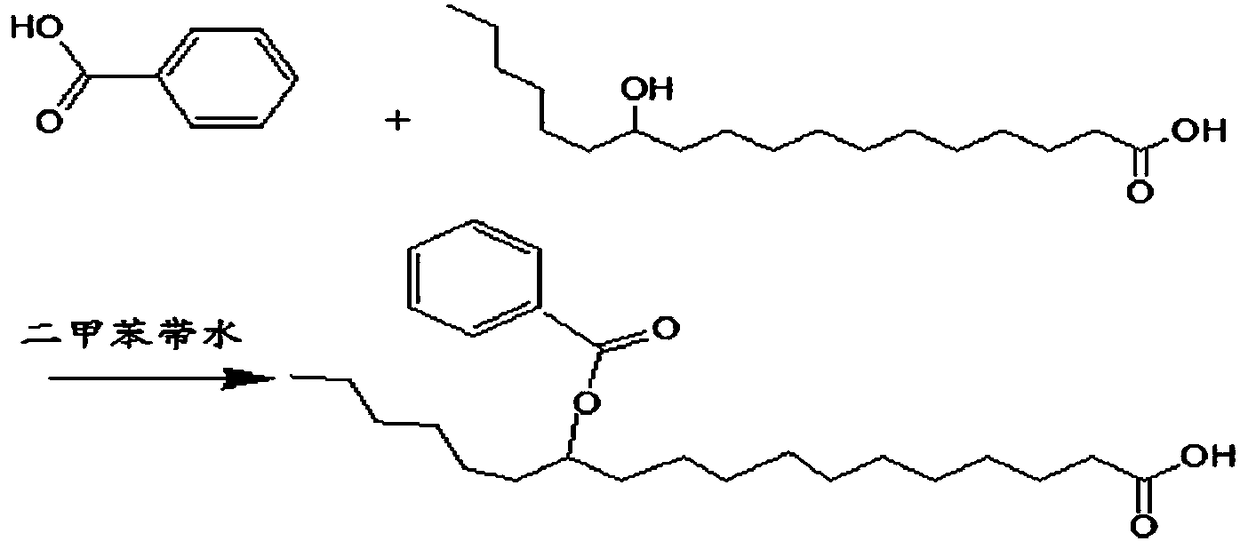
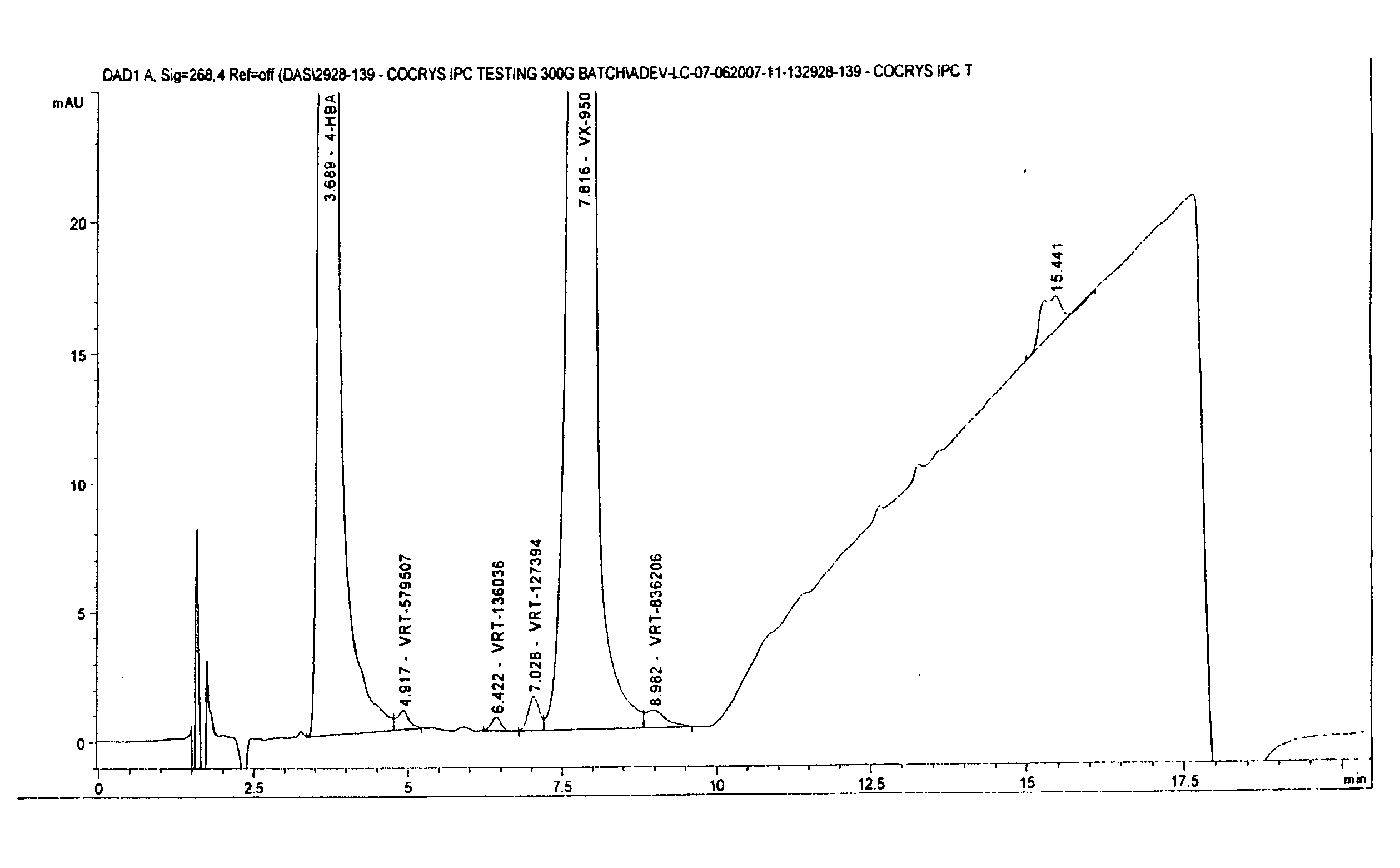
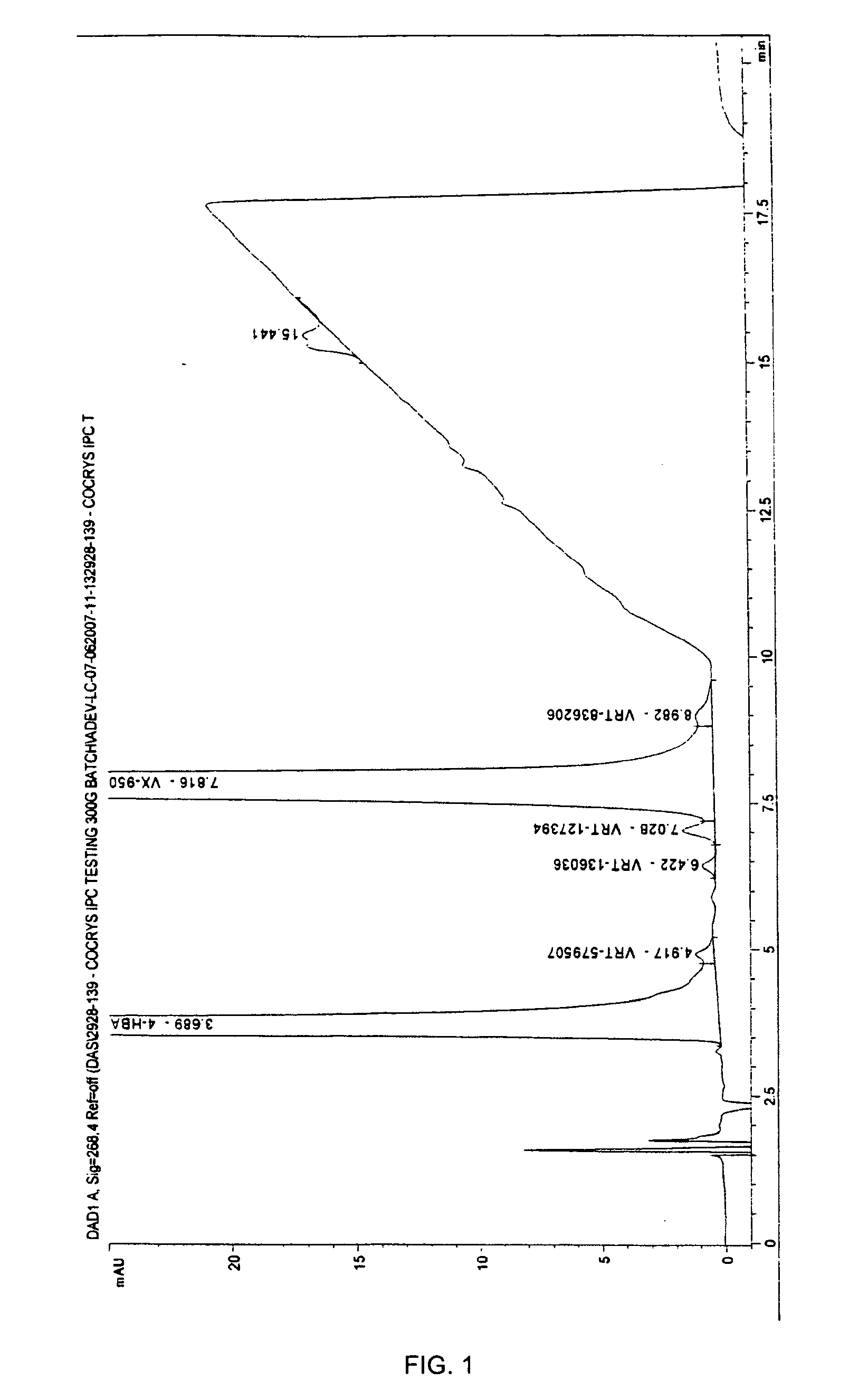
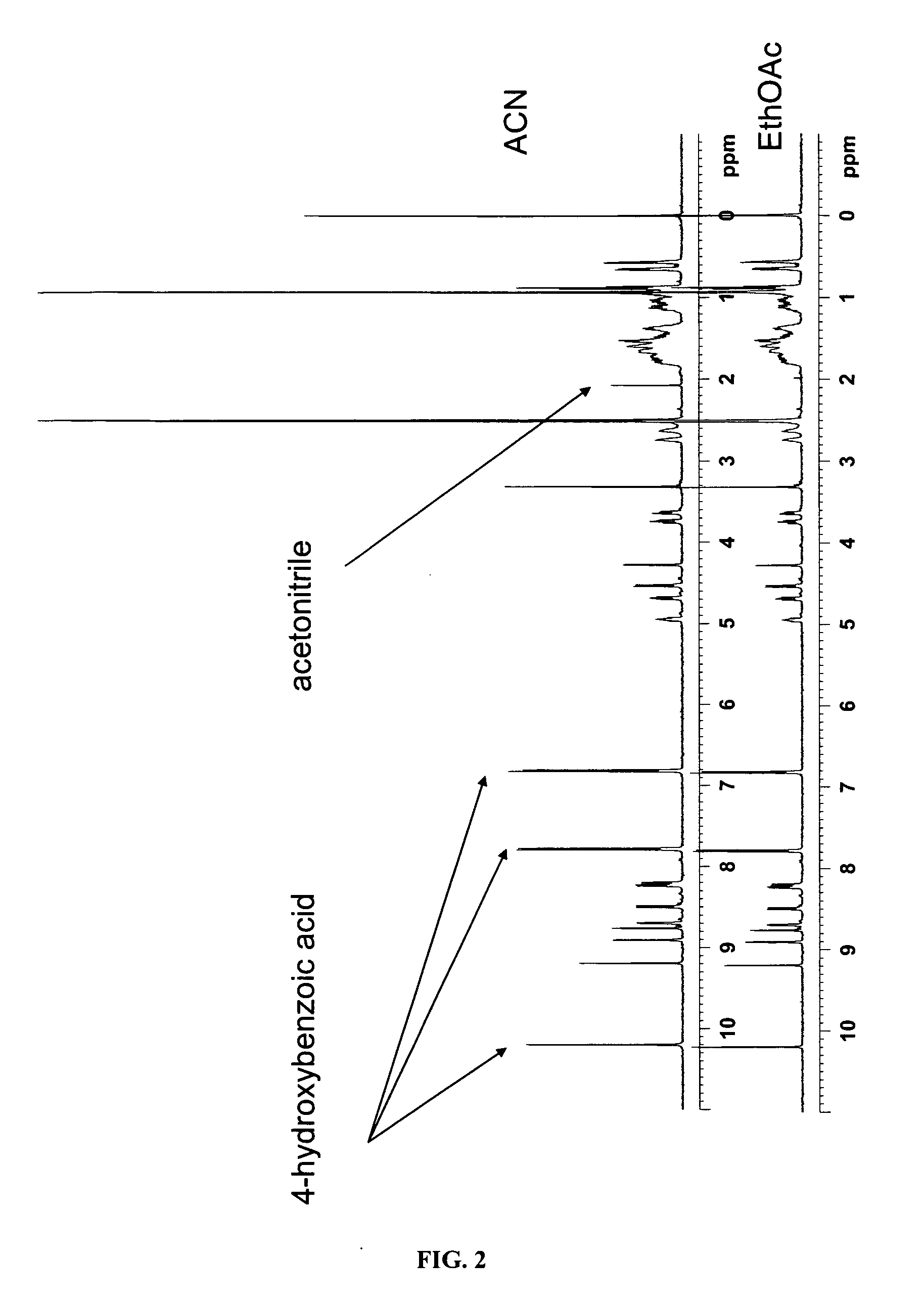
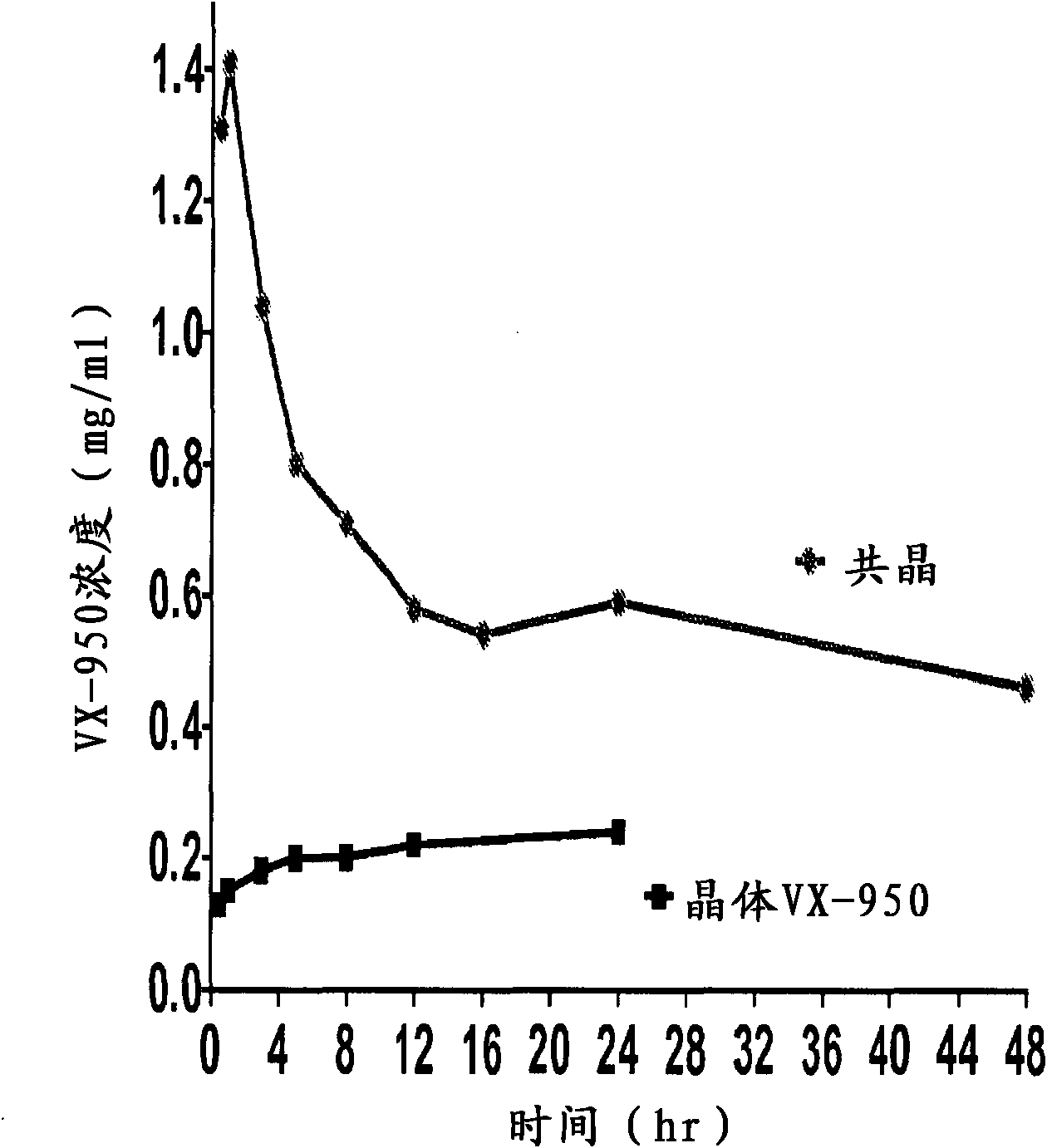
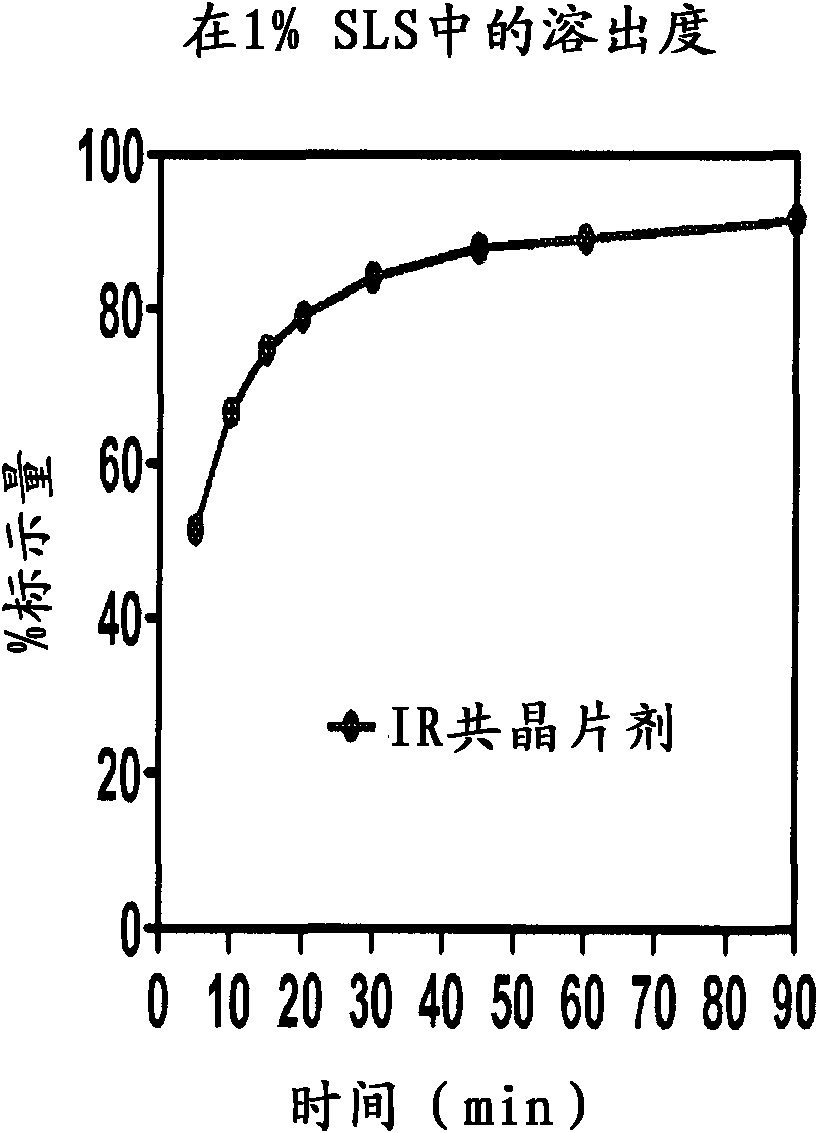
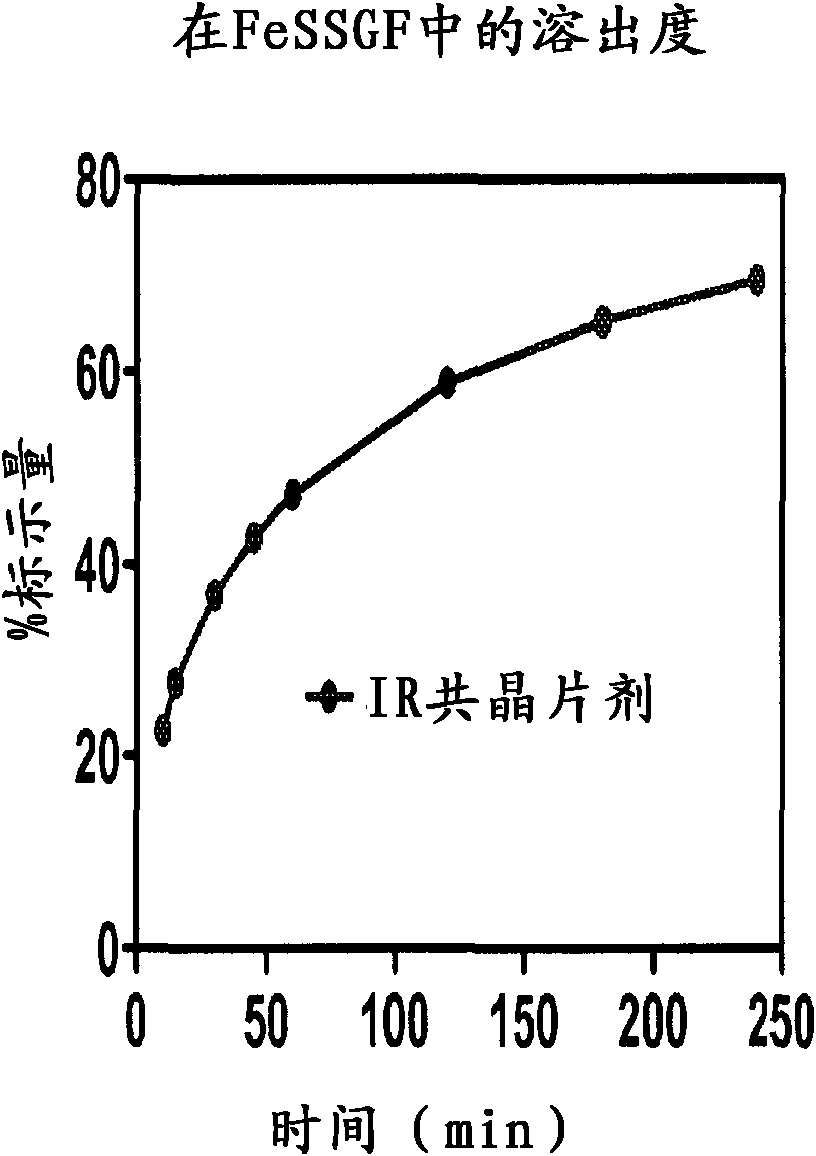
Co-Crystals and Pharmaceutical Compositions Comprising the Same
InactiveUS20110059987A1High dissolution rateGood water solubilityOrganic active ingredientsTetrapeptide ingredientsSalicylic acidAdipic acid
The invention relates to compositions and co-crystals each comprising VX-950 and a co-crystal former selected from the group consisting of 4-hydroxybenzoic acid, 4-amino salicylic acid, phenylalanine, threonline, tartaric acid, adipic acid, succinic acetate, proline, methyl 4-hydroxybenzoate, anthranilic acid, and d-Biotin. Also within the scope of this invention are methods of making and using the same.
Owner:VERTEX PHARMA INC
Method for synthesizing 3-geranyl-4-hydroxybenzoic acid and xiamenmycin by utilizing escherichia coli
ActiveCN108865961AIncrease productionAchieve biosynthesisBacteriaMicroorganism based processesEscherichia coliGeranyl pyrophosphate
The invention discloses a method for synthesizing 3-geranyl-4-hydroxybenzoic acid and xiamenmycin by utilizing escherichia coli. According to the method, an adopted escherichia coli strain contains xiamenmycin synthesis related genes and mevalonic acid pathway related genes which are partially or entirely subjected to codon optimization; three genes required by synthesis of the xiamenmycin are imported into the escherichia coli; the three genes are subjected to inducible expression, and GBA and the xiamenmycin are synthesized in the escherichia coli; the feed of intracellular geranyl pyrophosphate of the escherichia coli is further improved by introducing a mevalonic acid synthesis pathway, and the yield of the GBA is further increased by adding 4-hydroxybenzoic acid into a fermentation medium; the yield of the synthesized 3-geranyl-4-hydroxybenzoic acid reaches 94 mg / L, and the yield of the xiamenmycin reaches 11 mg / L; and the biosynthesis of the 3-geranyl-4-hydroxybenzoic acid and the xiamenmycin in the escherichia coli is realized for the first time.
Owner:SHANGHAI JIAO TONG UNIV
Preparation of 4-hydroxybenzoyl chloride
InactiveCN101376627ALow purityHigh yieldOrganic compound preparationCarboxylic compound preparationBenzeneDistillation
The invention discloses a method for preparing 4-hydroxybenzoyl chloride. The method comprises the following steps: (1) adding 4-hydroxybenzoic acid, a benzene solvent and N,N-dimethyl formamide as an cosolvent into a container, heating up to 30-65 DEG C while stirring, slowly and dropwise adding thionyl chloride for 0.5 to 1 hour and reacting at 30 to 65 DEG C for 2 to 5 hour; (2) standing to demix the reaction solution; and (3) respectively recovering thionyl chloride, benzene and N,N-dimethyl formamide respectively by distilling and distilling under the reduced pressure, and concentrating the benzene layer solution to separate viscose solid (4-hydroxybenzoyl chloride). The method is simple and reliable, and by adopting the method, 4-hydroxybenzoyl chloride with high purity and high yield can be obtained without need of the reduced pressure distillation.
Owner:SHANGHAI INST OF TECH
Amorphous wholly aromatic polyester amide composition
InactiveCN1732229AImprove adhesionImprove mechanical propertiesSynthetic resin layered productsThin material handlingPolyesterPolymer science
An amorphous, wholly aromatic polyesteramide composition which is excellent in stretchability and adhesion to different kinds of polymers. The composition is obtained by incorporating 1 to 30 wt.% modified polyolefin resin or polyamide resin which has a melting point of 230 DEG C or lower or is amorphous into a wholly aromatic amorphous polyesteramide which becomes optically anisotropic upon softening / fluidization and is obtained by copolymerizing (A) 4-hydroxybenzoic acid, (B) 2-hydroxy-6-naphthoic acid, (C) 7 to 35 mol% aromatic aminophenol (based on all monomers), and (D) one or more aromatic dicarboxylic acids 35 mol% or more of which are accounted for by isophthalic acid, provided that the ingredient (C) may be replaced by (C)' 3 to 15 mol% aromatic diamine (based on all monomers), the proportion of flexible monomers in all starting monomers being 7 to 35 mol% and the (A) / (B) ratio being from 0.15 to 4.0, and which has a glass transition temperature of 100 to 180 DEG C and gives no melting point when analyzed by DSC.
Owner:POLYPLASTICS CO LTD
Co-crystals and pharmaceutical formulations comprising the same
InactiveCN102215816AAntiviralsPharmaceutical non-active ingredientsImmediate releaseBULK ACTIVE INGREDIENT
An immediate release formulation of an active ingredient, wherein the active ingredient is a co-crystal of VX-950 and a 4-hydroxybenzoic acid.
Owner:VERTEX PHARMA INC
Application of Bacillus in degrading aromatic compounds
InactiveCN106698678ARich diversityImprove degradation efficiencyWater contaminantsWaste water treatment from textile industryBenzoic acidMicroorganism
The invention provides application of Bacillus in degrading aromatic compounds, belonging to the technical field of microbes. The experiment proves that the Bacillus L1 strain has the capacity for degrading 3,4-dihydroxybenzoic acid, 3-methoxy-4-hydroxybenzaldehyde, catechol and other environmental pollutants; the degradation rates for para-hydroxybenzoic acid, 3,4-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid, 3-methoxy-4-hydroxybenzoic acid, 3-methoxy-4-hydroxybenzaldehyde, catechol and benzoic acid with the concentration of 500 mg / L can respectively reach 90% or above; and thus, the Bacillus L1 strain has wide application prospects in the aspect of environmental treatment.
Owner:JIANGSU UNIV
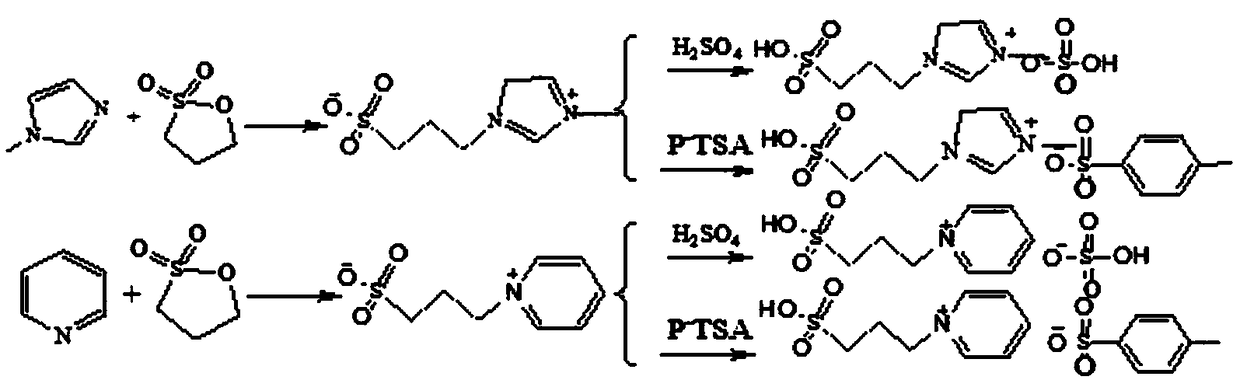
Method for catalytic synthesis of 3,5-ditertbutyl-4-hydroxybenzoic acid n-hexadecyl ester by acidic ionic liquid
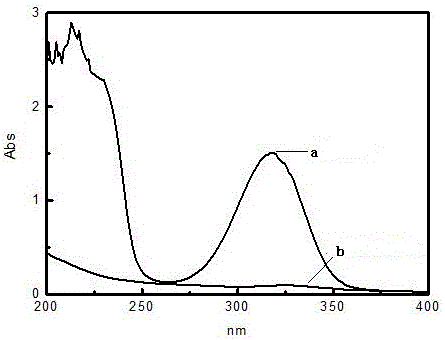
ActiveCN108218699AReduce generationEnvironmentally friendlyOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsChemical industryDual core
The invention discloses a method for catalytic synthesis of 3,5-ditertbutyl-4-hydroxybenzoic acid n-hexadecyl ester by acidic ionic liquid, and belongs to the field of chemical industry. By adoption of the method, the problems of complex process flow, troublesome aftertreatment and pollution to the environment of the existing method are solved. The method comprises the following steps: by utilizing acidic dual-core functional ionic liquid as a catalyst, and adopting 3,5-ditertbutyl-4-hydroxybenzoic acid and n-hexadecyl alcohol as raw materials, utilizing vacuumization for dewatering, controlling reaction temperature to be 100-160 DEG C and reacting for 3-8 hours, after reaction is ended, cooling, and standing for layering, separating out a lower layer namely ionic liquid to repeatedly usethe same; pouring an upper layer into methanol solution to generate a large amount of crystals, and filtering to obtain filter cakes, namely a target product. The method disclosed by the invention adopts the ionic liquid as a pollution-free catalyst and shows the advantages of high efficiency, high selectivity, mild reaction condition, simple and controllable effects, environment friendliness, high catalytic efficiency, simple aftertreatment and the like in various acid catalytic reactions.
Owner:兰州精细化工有限责任公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com