Production method of 3,5-di-tert-butyl-4-hydroxybenzoic acid
A technology of hydroxybenzoic acid and di-tert-butylphenol, which is applied in the direction of chemical instruments and methods, carboxylate preparation, carboxylate preparation, etc., can solve the problems of easy coking of products, increased cost, and difficult industrialization of the process, so as to avoid Effects of local overheating and coking, reducing equipment cost and increasing product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1, a kind of preparation technology of 3,5-di-tert-butyl-4-hydroxybenzoic acid with toluene as solvent, carries out the following steps successively:
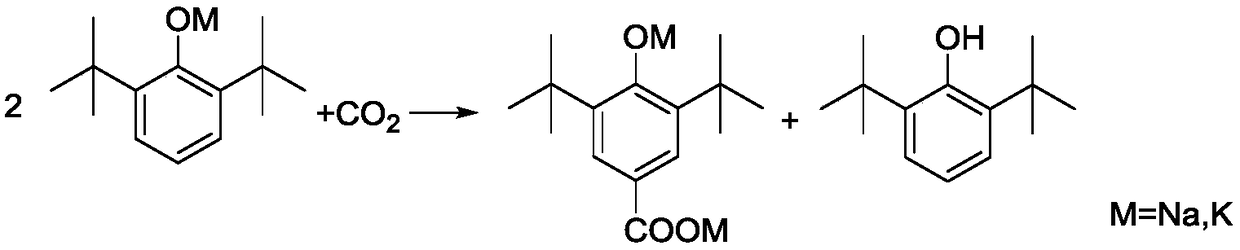
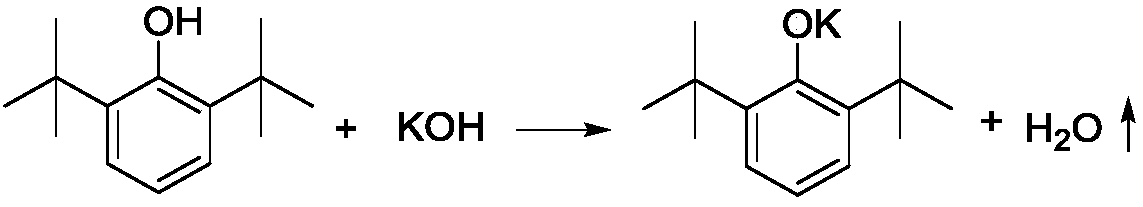
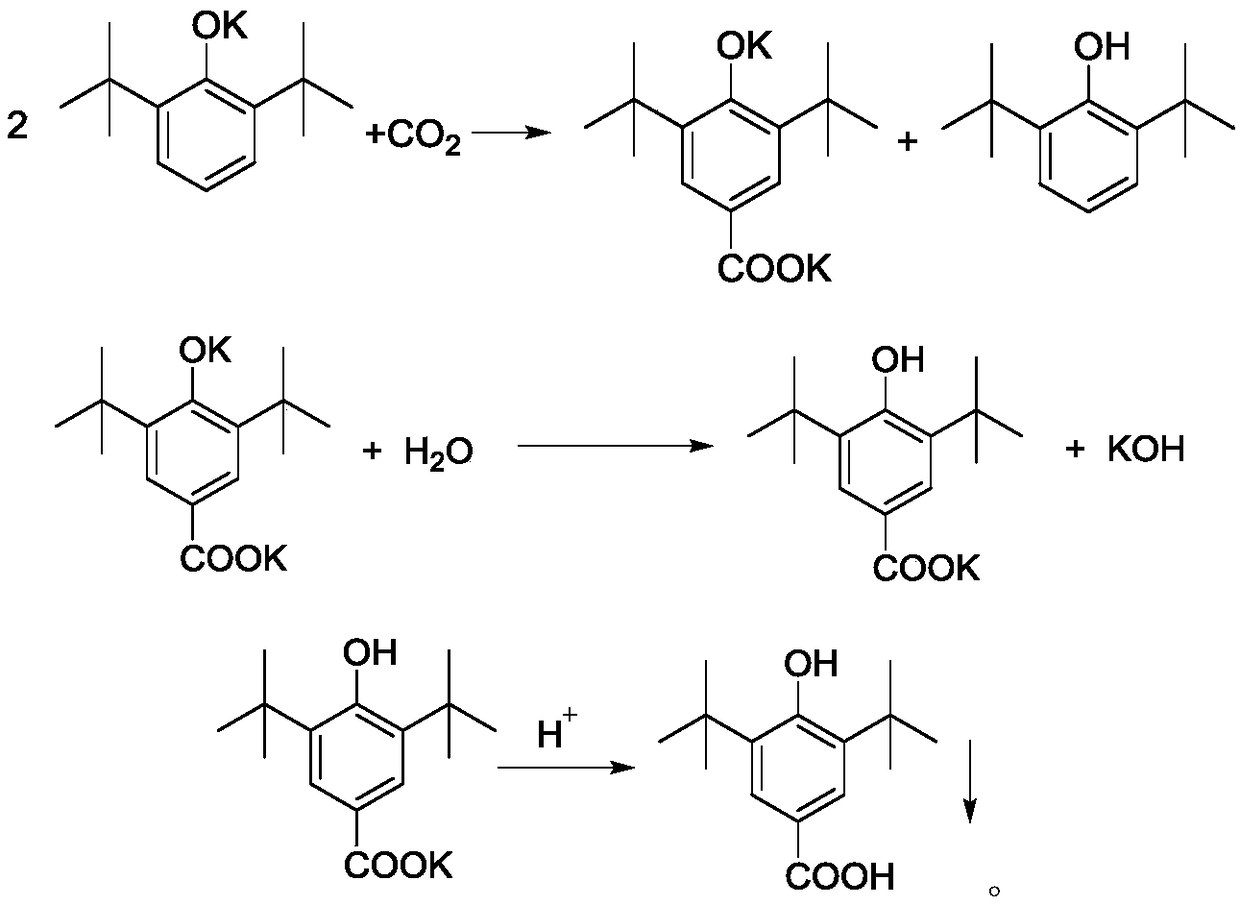
[0049] A. Add 412g 2,6-di-tert-butylphenol (2mol), 50% (mass) potassium hydroxide aqueous solution 235g (2.1mol KOH), toluene 920g (10mol) to the reaction kettle protected with nitrogen, and the reaction kettle There is a water separator with cold hydrazine on it, which is used to reflux toluene and discharge water. After reacting for 4 hours under nitrogen protection and reflux conditions, the reaction product is a suspension of potassium phenate and toluene;
[0050] B. After the potassium phenate preparation is completed, close the water-distributing equipment, feed dry carbon dioxide (that is, moisture content≤0.01%) in the kettle, and the pressure of carbon dioxide is 2.5MPa, and keep continuously feeding carbon dioxide in the kettle to Keep the pressure inside the kettle stable;
[0051] C. Heat the re...
Embodiment 2
[0055] Embodiment 2, a kind of preparation technology of 3,5-di-tert-butyl-4-hydroxybenzoic acid with toluene as solvent, carries out the following steps successively:
[0056] A. Add 618g 2,6-di-tert-butylphenol (3mol), 60% (mass) potassium hydroxide aqueous solution 309g (3.3mol KOH), toluene 2760g (30mol) to the reaction kettle protected by nitrogen, and the reaction kettle There is a water separator with cold hydrazine on it, which is used to reflux toluene and discharge water. After reacting for 3 hours under nitrogen protection and reflux conditions, the reaction product is a suspension of potassium phenate and toluene;
[0057] B. After the preparation of potassium phenolate is completed, close the water-distributing equipment, feed dry carbon dioxide into the kettle, the pressure of carbon dioxide is 1.5MPa, and keep feeding carbon dioxide continuously into the kettle to maintain the pressure stability in the kettle;
[0058] C. Heat the reactor to 100°C, react for 8 ...
Embodiment 3
[0062] Embodiment 3, a kind of preparation technology that takes toluene as solvent 3,5-di-tert-butyl-4-hydroxybenzoic acid, carries out the following steps successively:
[0063] A. Add 206g 2,6-di-tert-butylphenol (1mol), 147g (1.05mol KOH) of 40% (mass) potassium hydroxide aqueous solution, 736g (8mol) of toluene to the reactor that has been protected with nitrogen, and the reactor There is a water separator with cold hydrazine on it, which is used to reflux toluene and discharge water. After reacting for 2 hours under nitrogen protection and reflux conditions, the reaction product is a suspension of potassium phenate and toluene;
[0064] B. After the potassium phenol salt is prepared, close the water-distributing equipment, feed dry carbon dioxide into the kettle, the pressure of carbon dioxide is 2.0MPa, and keep feeding carbon dioxide into the kettle continuously to maintain the pressure stability in the kettle;
[0065] C. Heat the reactor to 120°C, react for 10 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com