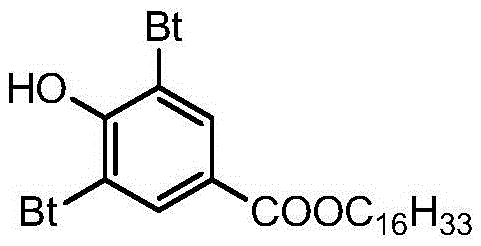
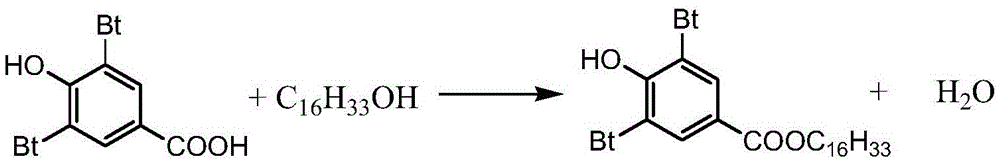
Preparing method for 3,5-bis(tertiary butyl)-4-hydroxybenzoic acid hexadecane alkyl ester
A technology of n-hexadecyl hydroxybenzoate and hydroxybenzoic acid, which is applied in the field of preparation of n-hexadecyl hydroxybenzoate, can solve solvent distillation recovery energy Consumption, environmental pollution, solvent loss and other problems, to achieve the effect of saving solvent consumption and energy consumption, reducing production costs, and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 100g (0.4mol) of 3,5-di-tert-butyl-4-hydroxybenzoic acid, n-deca 290.4 g (1.2 mol) of hexaol and 10 g of p-toluenesulfonic acid as a catalyst were introduced into a nitrogen stream, the temperature was raised to 90° C., the pressure was 10 kPa and the temperature was maintained for 10 h. The end point of the reaction was monitored by TLC. After the reaction, the reaction solution was cooled to 70-75°C, and then the reaction solution was added to saturated aqueous sodium carbonate solution (50mL) (to remove the catalyst and unreacted raw material 3,5-di-tert-butyl-4-hydroxybenzoic acid ), then washed with water until the pH of the water layer was 6.5 to 7, then took the organic layer and evaporated the residual moisture under reduced pressure to obtain a crude product, which was recrystallized with anhydrous methanol, filtered, and the filter cake was dried to obtain White crystalline solid product light stabilizer 3,5-di-tert-butyl-4-hydroxybenzoic acid n-hexadecyl ...
Embodiment 2
[0026] Add 100g (0.4mol) of 3,5-di-tert-butyl-4-hydroxybenzoic acid, n-deca 96.8 g (0.4 mol) of hexanol and 5.0 g of sodium bisulfate were passed through a stream of carbon dioxide, the temperature was raised to 90° C., and the pressure was kept at 6 kPa for 8 hours. The end point of the reaction was monitored by TLC. After the reaction, the reaction solution was cooled to 70-75°C, and then the reaction solution was added to a saturated sodium carbonate aqueous solution (20mL) (to remove the catalyst and unreacted raw material 3,5-di-tert-butyl-4-hydroxybenzoic acid ), then washed with water until the pH of the water layer was 6.5 to 7, then took the organic layer and evaporated the residual moisture under reduced pressure to obtain a crude product, which was recrystallized with anhydrous methanol, filtered, and the filter cake was dried to obtain White crystalline solid product light stabilizer 3,5-di-tert-butyl-4-hydroxybenzoic acid n-hexadecyl ester 175.4g, yield reaches 92...
Embodiment 3
[0028] Add 100g (0.4mol) of 3,5-di-tert-butyl-4-hydroxybenzoic acid, n-deca 145.2 g (0.6 mol) of hexaol and 8.0 g of polyphosphoric acid were passed through a stream of carbon dioxide, the temperature was raised to 140° C., and the pressure was kept at 10 kPa for 8 hours. The end point of the reaction was monitored by TLC. After the reaction, the reaction solution was cooled to 70-75°C, and then the reaction solution was added to saturated aqueous sodium bicarbonate solution (50mL) (to remove the catalyst and unreacted raw material 3,5-di-tert-butyl-4-hydroxybenzene formic acid), then washed with water until the pH of the water layer was 6.5-7, then the organic layer was evaporated under reduced pressure to remove the residual moisture to obtain a crude product, which was recrystallized with anhydrous methanol, filtered, and the filter cake was dried Obtain white crystalline solid product light stabilizer 3,5-di-tert-butyl-4-hydroxyl benzoic acid n-hexadecyl ester 170.3g, yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com