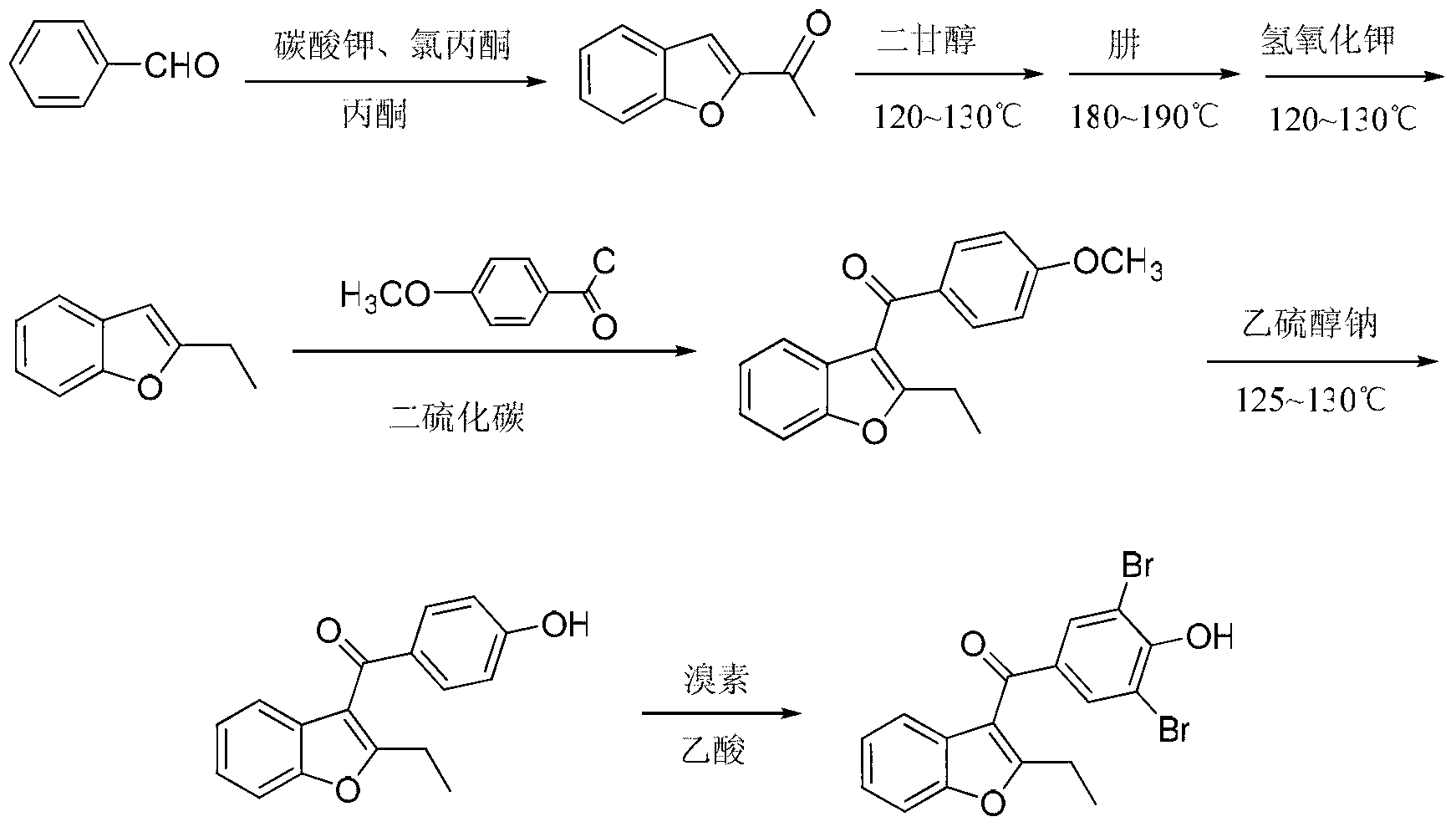
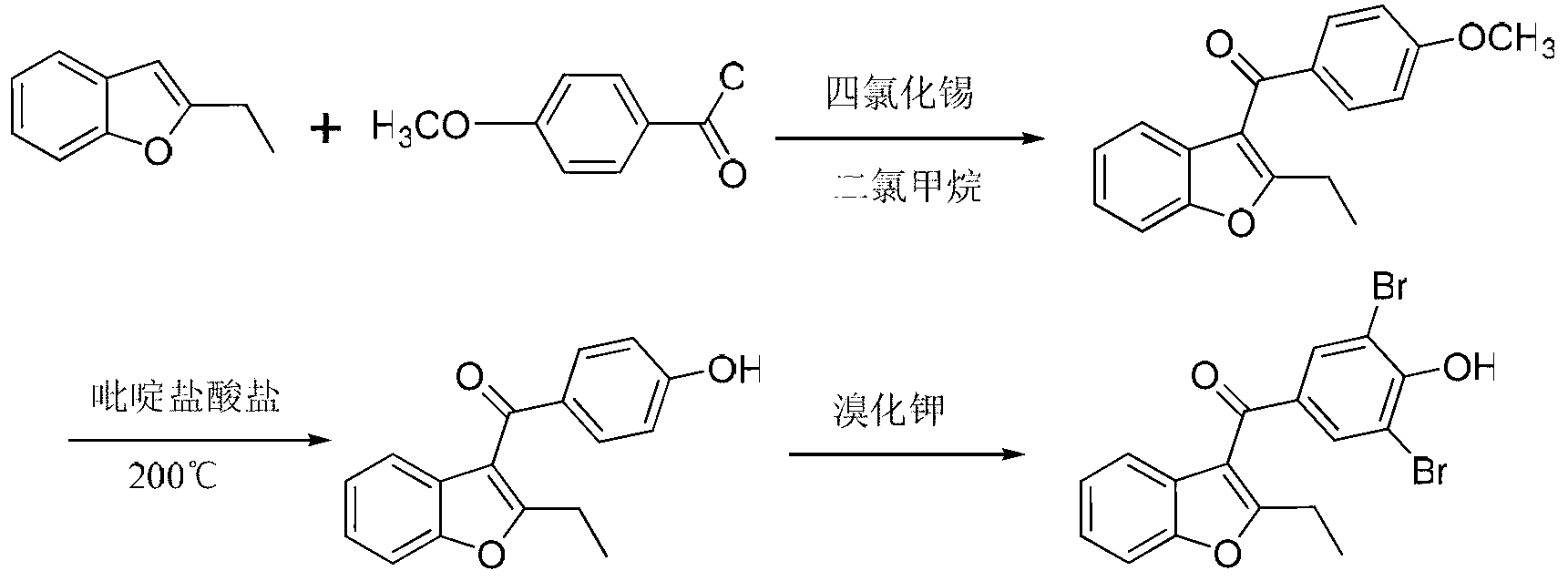
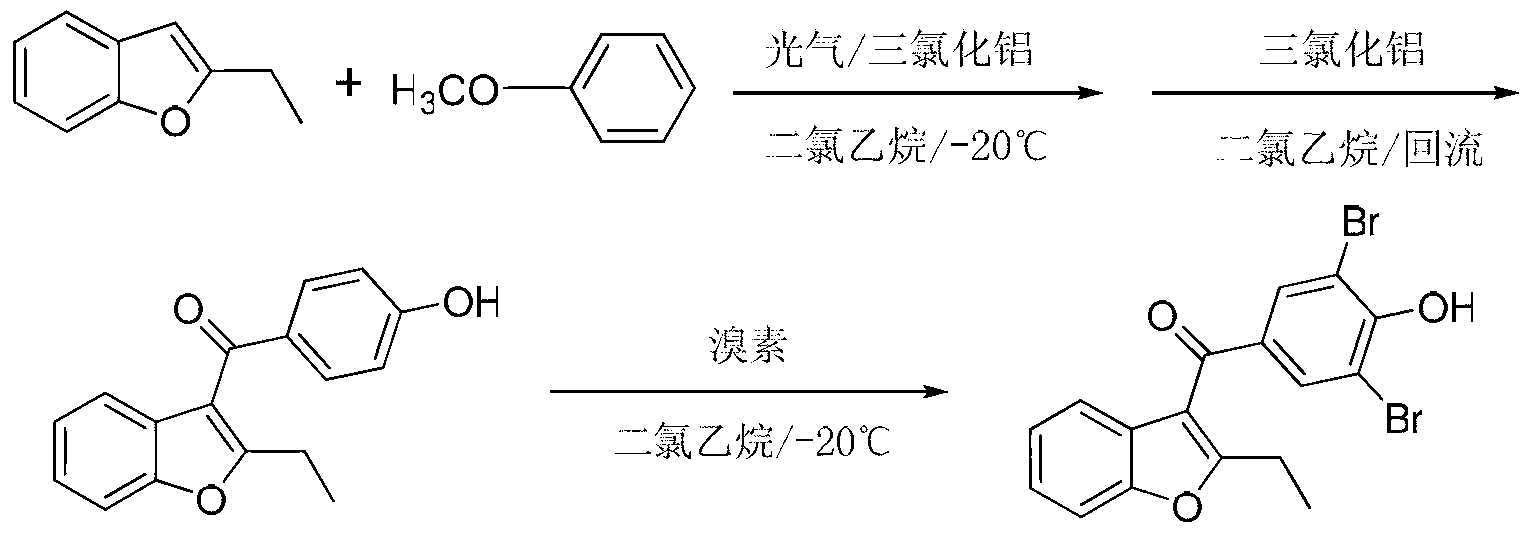
Method for preparing benzbromarone
A technology of benzbromarone and catalyst, which is applied in the field of pharmaceutical chemical synthesis, and can solve problems that are unfavorable to labor protection and unfavorable industrialized production of benzbromarone.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of 3,5-dibromo-4-acetoxybenzoic acid (III):
[0024] Add 125ml of dichloromethane and 3.67g of acetic anhydride to the reaction flask, add 10g of 3,5-dibromo-4-hydroxybenzoic acid (II) while stirring, add 5-6 drops of concentrated sulfuric acid, and control the temperature at 30-35°C. React for 2 hours, add 250ml of saturated sodium chloride solution to the reaction flask, stir for 1 hour, let stand to separate layers, discard the water layer, wash the organic layer with saturated sodium chloride until neutral, dry the organic layer with anhydrous magnesium sulfate, filter Inorganic salt, and the filtrate was concentrated to dryness to obtain 10.4 g of 3,5-dibromo-4-acetoxybenzoic acid (III) as a white powder solid, with a yield of 91.23%.
[0025] Melting point: 194~196℃
[0026] 1 H NMR data: (400MHz, DCl 3 , 25°C) δ 2.43 (s, 3H), 8.29 (s, 2H), 11.03 (s, 1H) ppm.
[0027] Preparation of 3,5-dibromo-4-acetoxybenzoyl chloride (IV):
[0028] Add 10g of 3...
Embodiment 2
[0036] Preparation of 3,5-dibromo-4-acetoxybenzoic acid (III):
[0037] Add 50ml of dichloromethane and 5.5g of acetic anhydride to the reaction flask, add 5g of 3,5-dibromo-4-hydroxybenzoic acid (II) while stirring, add 2-3 drops of concentrated sulfuric acid, and control the temperature at 30-35°C. React for 2 hours, add 100ml of saturated sodium chloride solution to the reaction bottle, stir for 1 hour, let stand to separate layers, discard the water layer, wash the organic layer with saturated sodium chloride until neutral, dry the organic layer with anhydrous magnesium sulfate, filter Inorganic salt, the filtrate was concentrated to dryness to obtain 4.62 g of 3,5-dibromo-4-acetoxybenzoic acid (III) as a white powder solid, with a yield of 81%.
[0038] Preparation of 3,5-dibromo-4-acetoxybenzoyl chloride (IV):
[0039] Add 4g of 3,5-dibromo-4-acetoxybenzoic acid (III) and 70ml of thionyl chloride to the reaction flask in sequence, and add a drop of DMF dropwise, and sti...
Embodiment 3
[0045] Preparation of 3,5-dibromo-4-acetoxybenzoic acid (III):
[0046] Add 80ml of dichloromethane and 8g of 3,5-dibromo-4-hydroxybenzoic acid (II) into the reaction flask, add 2-3 drops of concentrated sulfuric acid, control the temperature at 30-35°C, add 10.6g of acetyl chloride dropwise, and react 2h, add 200ml of saturated sodium chloride solution to the reaction flask, stir for 1h, let stand to separate layers, discard the water layer, wash the organic layer with saturated sodium chloride until neutral, dry the organic layer with anhydrous magnesium sulfate, filter out the inorganic salt, and the filtrate was concentrated to dryness to obtain 7.1 g of 3,5-dibromo-4-acetoxybenzoic acid (III) as a white powder solid, with a yield of 77.7%. Preparation of 3,5-dibromo-4-acetoxybenzoyl chloride (IV):
[0047] Add 7g of 3,5-dibromo-4-acetoxybenzoic acid (III) and 150ml of thionyl chloride to the reaction flask in sequence, and add a drop of DMF dropwise, and stir for 0.5 hou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com