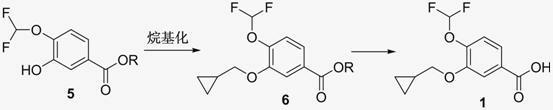
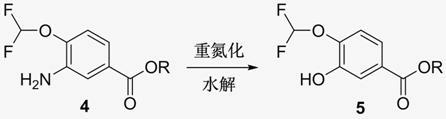
New method for synthesizing 3-cyclopropyl methoxy-4-(difluoromethoxy) benzoic acid
A technology of difluoromethoxybenzoic acid and cyclopropylmethoxy, which is applied in the preparation of carboxylate/lactone, organic chemistry, etc., can solve problems such as difficult industrial scale-up production, and achieve easy operation and low side reaction little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid
[0036] Step (1): Preparation of 3-nitro-4-difluoromethoxybenzoic acid methyl ester
[0037] 26.0g of methyl 3-nitro-4-hydroxybenzoate, 0.67g of benzyltrimethylammonium chloride, 31.4g of 50% sodium hydroxide and 200mL of dioxane were added to the reactor, and the Under stirring, chlorodifluoromethane was introduced into the system. After the reaction, ice water was added, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to obtain 28.4 g of methyl 3-nitro-4-difluoromethoxybenzoate with a yield of 87 %.
[0038] Step (2): Preparation of 3-amino-4-difluoromethoxybenzoic acid methyl ester
[0039] 28.0 g of methyl 3-nitro-4-difluoromethoxybenzoate was dissolved in 500 mL of methanol, 2.8 g of 10% palladium carbon (containing 50% water) was added, and hydrogenated at normal pressure for 10 h. Palladium carbon was removed by filtration...
Embodiment 2
[0045] Example 2: 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid
[0046] Step (1): Preparation of ethyl 3-nitro-4-difluoromethoxybenzoate
[0047] Add 35.0g of ethyl 3-nitro-4-hydroxybenzoate, 3.5g of tetrabutylammonium bromide, 65mL of 35% sodium hydroxide and 250mL of toluene into the reactor. into chlorodifluoromethane. After the reaction, ice water was added, the organic phase was separated, dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to obtain 36.8 g of ethyl 3-nitro-4-difluoromethoxybenzoate with a yield of 85%. .
[0048] Step (2): Preparation of ethyl 3-amino-4-difluoromethoxybenzoate
[0049] 36.0 g of ethyl 3-nitro-4-difluoromethoxybenzoate was dissolved in 500 mL of glacial acetic acid, 63.0 g of iron powder was added in batches at room temperature, and stirred for another 3 h. Add 500mL of toluene, filter through celite, and concentrate the filtrate. The residue was dissolved in ethyl acetate, washed with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com