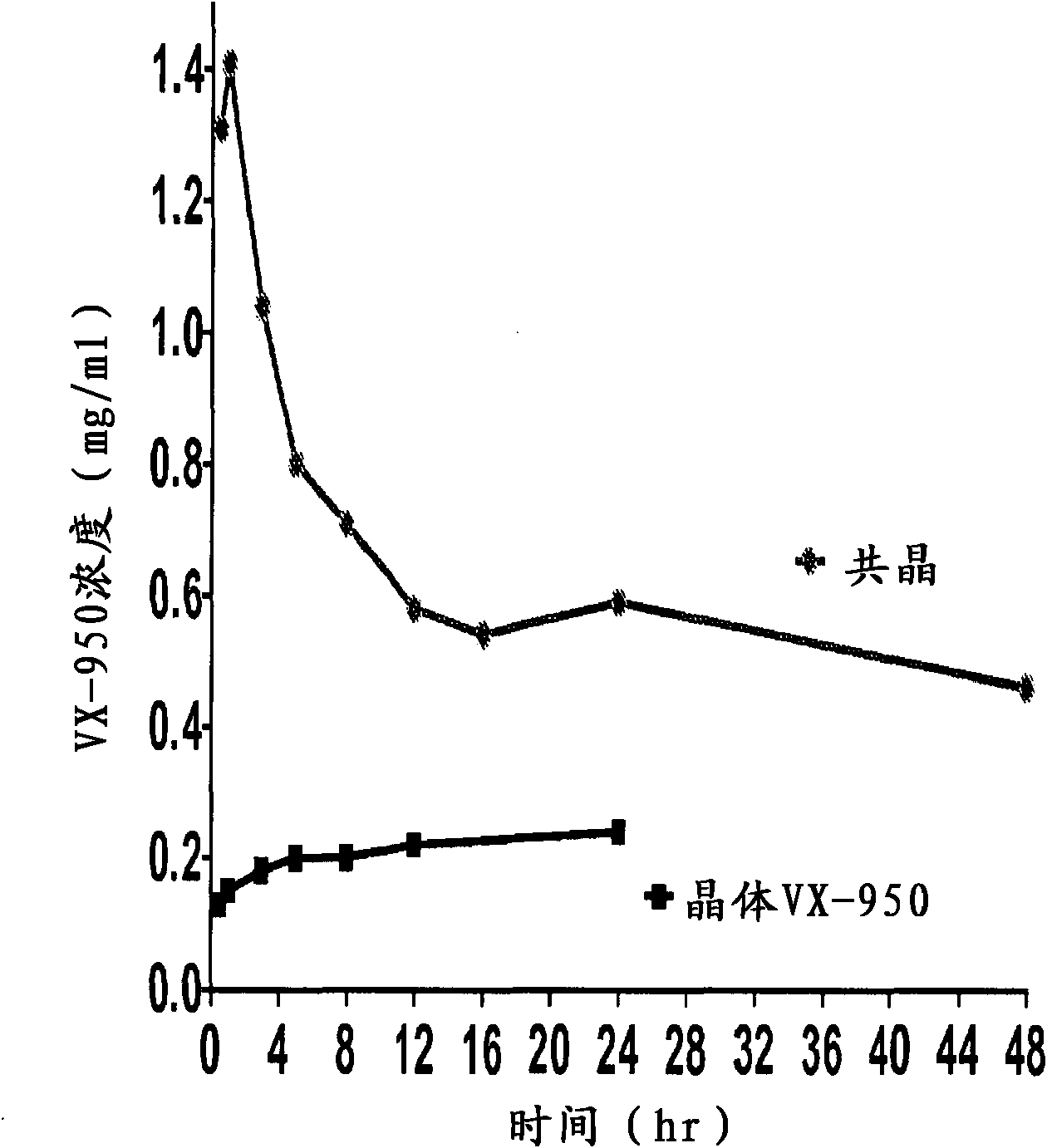
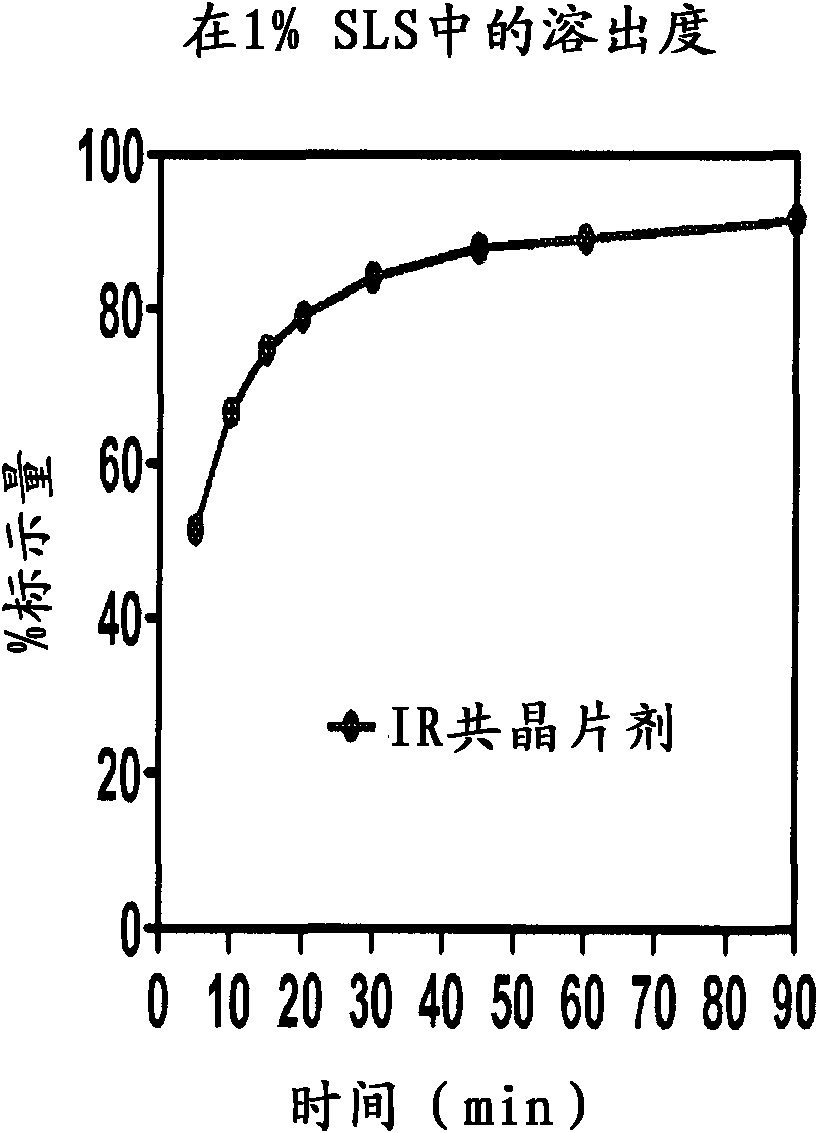
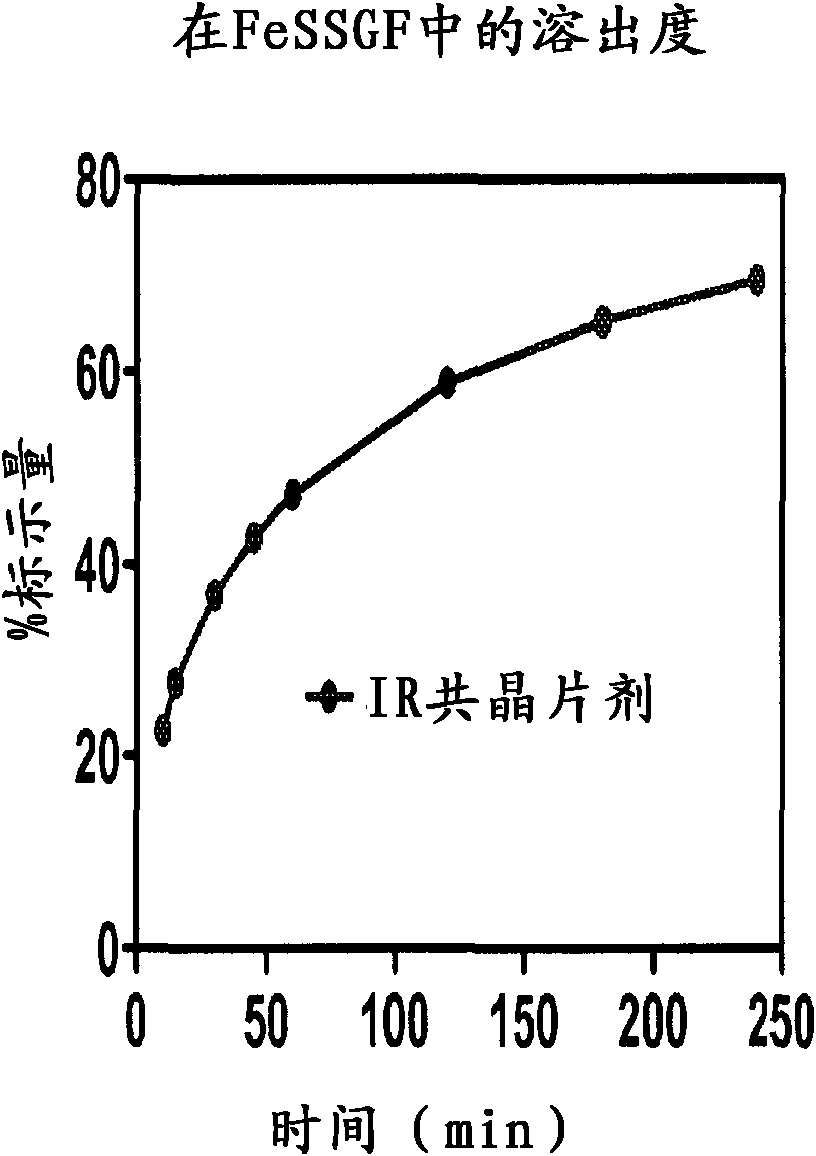
Co-crystals and pharmaceutical formulations comprising the same
A preparation and drug technology, applied in the field of co-crystals and pharmaceutical preparations containing the co-crystals, can solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0117] Example #1
[0118] Preparation of VX-950 and 4-Hydroxybenzoic Acid Cocrystals by Crystallization from Dichloromethane and tert-Butyl Methyl Ether Solutions Using the Co-Addition Method
[0119] A slurry of VX-950 / 4-hydroxybenzoic acid co-crystal (3 wt %) in 3:5 dichloromethane:tert-butyl methyl ether (20 mL) was added to a jacketed reactor with a temperature of 25 °C . A solution of VX-950 in dichloromethane (11.14 in 33.75 mL) and a solution of 4-hydroxybenzoic acid in tert-butyl methyl ether (2.5 g in 56.25 mL) were prepared in separate flasks. These solutions were then added to the reactor containing the eutectic slurry by separate pumps in appropriate proportions to maintain a 1:1.1 ratio of VX-950 to 4-hydroxybenzoic acid and a ratio of methylene chloride to tert-butyl methyl ether. The ratio is 3:5. The addition time was about 10 hours. The slurry was stirred for about 10-12 hours, then filtered. The filter cake was washed with 40 mL of 3:5 dichloromethane:t...
Embodiment 6
[0166] Preparation of VX-950 and 4-Hydroxybenzoic Acid Cocrystals by Crystallization from Dichloromethane, Tetrahydrofuran, and n-Heptane Solutions Using Antisolvent Addition
[0167] VX-950 (10.1 g, 14.9 mmol) and 4-hydroxybenzoic acid (2.27 g, 1.1 equiv) were added to a jacketed vessel maintaining the temperature at 22°C. A mixture of dichloromethane (45 mL) and tetrahydrofuran (15 mL) was then added to the vessel, and the contents were stirred until the two components were dissolved. Then 18 mL of the antisolvent n-heptane was added via a syringe pump at a rate of 30 mL / hour. The contents of the container remain in solution. Then VX-950 / 4-hydroxybenzoic acid co-crystal (300 mg, 3 wt% based on VX-950 loading) was added as seed crystals. The slurry was stirred for 45-60 minutes, during which time the eutectic seeds remained as a fine suspension. Another 8.1 mL of n-heptane was continuously added at a rate of about 4 mL / hour. The remainder of n-heptane (50.26 mL) was added...
Embodiment 7
[0178] Preparation of co-crystals of VX-950 and 4-hydroxybenzoic acid with particle size control during crystallization
[0179] A 30 g grade of VX-950 and 4-hydroxybenzoic acid co-crystal was prepared using a procedure similar to that described in Example #4, except that 20 wt% (based on the starting amount of VX-950) jet-milled seeds were added. This method yielded 36.98 g of co-crystal with 100% AUC purity. This amount is equivalent to an isolated yield of 87.6% taking into account the added seeds. The resulting co-crystal contained residual solvent levels of <100 ppm for dichloromethane and 446 ppm for tert-butyl methyl ether. The molar ratio of VX-950 to 4-hydroxybenzoic acid is 1:1.01. In contrast to the other batches, the resulting co-crystals consisted of small particles rather than thin rods. Particle size control can also be achieved by changing one or more processing parameters (eg, solvent type, addition time, inoculum size, inoculation point, etc.).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com