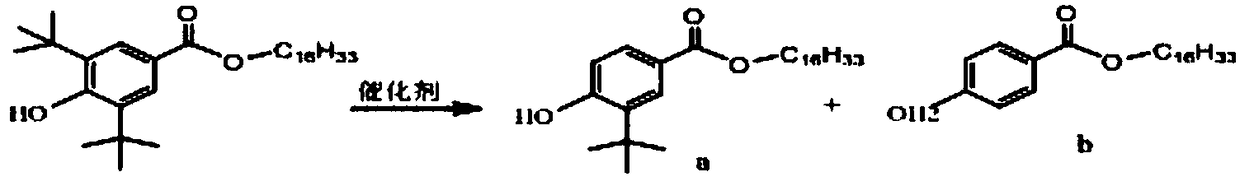
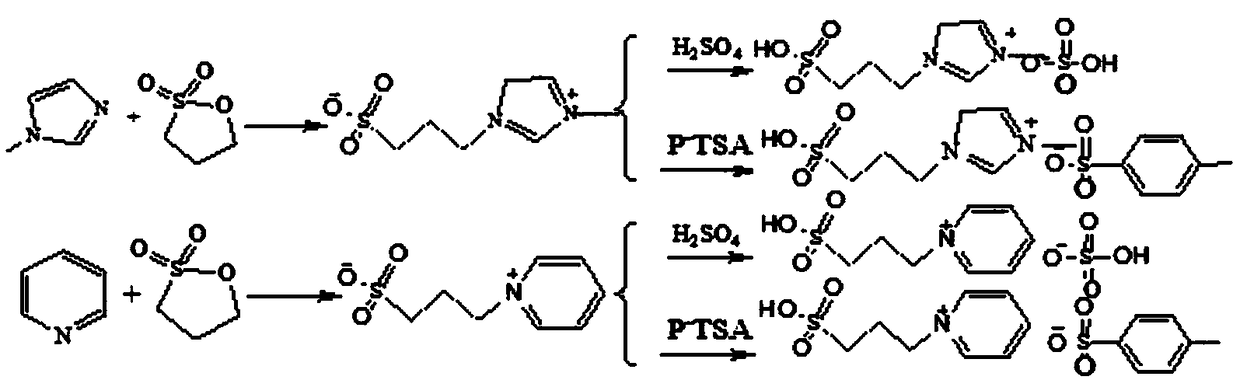
Method for catalytic synthesis of 3,5-ditertbutyl-4-hydroxybenzoic acid n-hexadecyl ester by acidic ionic liquid
An acidic ionic liquid, hydroxybenzoic acid technology, applied in the chemical industry, can solve the problems of complex process flow, strong corrosiveness of raw materials, easy generation of by-products, etc., and achieve the effects of simplified process, high catalytic efficiency and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Weigh 3,5-di-tert-butyl-4-hydroxybenzoic acid 31.25g (0.125mol), n-hexadecanol 30.25g (0.125mol), ionic liquid [HS0 3 -pmim] + [HS0 4 ] - 0.01mo1; first add the weighed n-hexadecanol into a three-necked flask equipped with a stirrer, a thermometer, and a reflux condenser, raise the temperature until the n-hexadecyl alcohol is completely dissolved, and then add the weighed 3,5-di-tert-butyl Base-4-hydroxybenzoic acid and ionic liquid catalyst were stirred, continued to heat up to 120° C., utilized vacuum dehydration (vacuum degree was -0.05 MPa), and kept the temperature for 5 hours. Use phase chromatography (HPLC method) to monitor when the reaction reaches the end point, cool the reaction system to 70°C, let it stand for stratification, the lower layer is an ionic liquid, and repeat the application after separation; the upper layer is cooled to 70°C, a large amount of crystallization appears after adding methanol solution, filter The obtained filter cake is the targ...
Embodiment 2
[0021] Weigh 3,5-di-tert-butyl-4-hydroxybenzoic acid 31.25g (0.125mol), n-hexadecanol 30.25g (0.125mol), ionic liquid [HS0 3 -pmim] + [pTSA] - 0.01mo1; first add the weighed cetyl alcohol into a three-necked flask equipped with a stirrer, a thermometer, and a reflux condenser, raise the temperature until the cetyl alcohol is completely dissolved, and then add 3,5-di-tert-butyl-4 -Hydroxybenzoic acid and ionic liquid catalyst are stirred, continue to heat up to 160 ℃, utilize vacuum dehydration (vacuum degree is-0.02MPa), heat preservation reaction 3 hours; Monitor reaction with phase chromatography (HPLC method) when reaching end point, cool down to Stand at 70°C for stratification, the lower layer is the ionic liquid, which can be used repeatedly after separation; the upper layer is added with methanol solution, a large number of crystals appear, and the filter cake obtained by filtration is the target product. The esterification rate was 98.6%, and the yield was 90.0%.
Embodiment 3
[0023] Weigh 3,5-di-tert-butyl-4-hydroxybenzoic acid 31.25g (0.125mol), n-hexadecanol 30.25g (0.125mol), ionic liquid [HS0 3 -pPy] + [HS0 4 ] - 0.019mol; First add the weighed n-hexadecanol into a three-necked flask equipped with a stirrer, a thermometer and a reflux condenser, raise the temperature until the n-hexadecanol is completely dissolved, then add 3,5-di-tert-butyl-4 -Hydroxybenzoic acid and ionic liquid catalyst, continue to stir and heat up to 100 ℃, utilize vacuum dehydration (vacuum degree is-0.08MPa), heat preservation reaction 8 hours; Stand at 70°C for stratification, the lower layer is the ionic liquid, which can be used repeatedly after separation; the upper layer is added with methanol solution, a large number of crystals appear, and the filter cake obtained by filtration is the target product. The esterification rate was 98.0%, and the yield was 90.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com