Organic fluorescent gel compound based on naphthalimide and preparation method and application thereof
A technology of hydroxynaphthimide and naphthalimide, which is applied in the field of supramolecular chemistry, can solve problems such as difficulties, complex synthesis, and difficult purification, and achieve the effect of sensitive detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
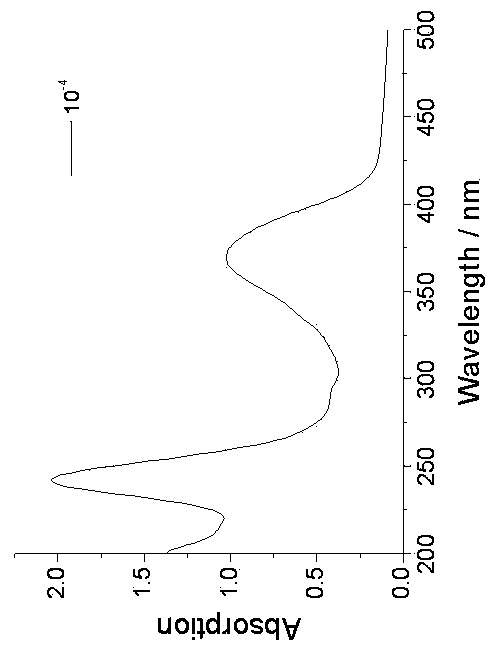
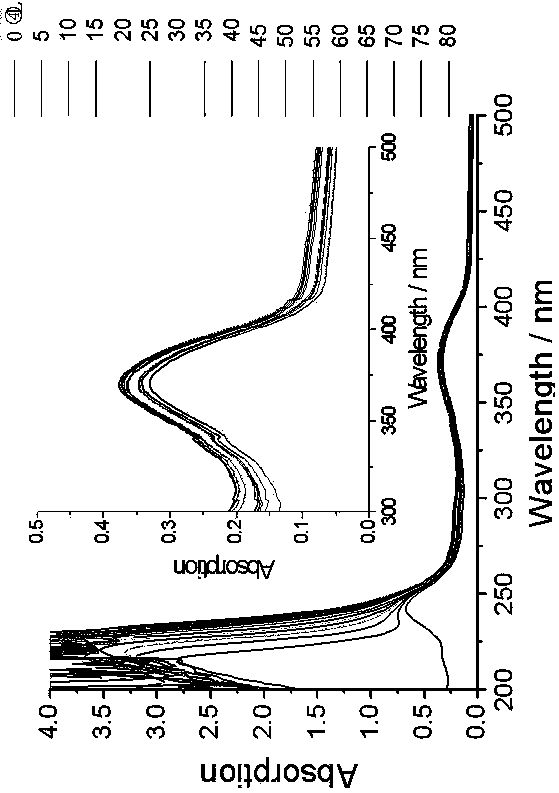
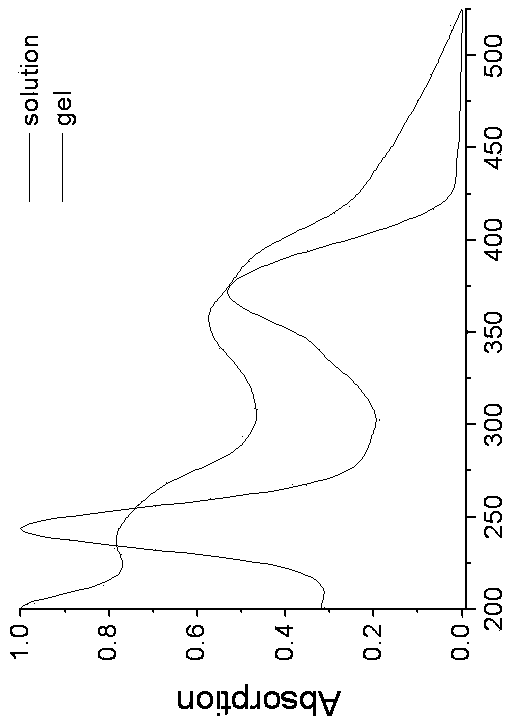
[0044] 4-Methoxy-N-hexanoyl-1,8-naphthalimide [4-CH3O-C12H5NO2-(CH2)5-COOH]: 4-bromo-p-phenylene-N-hexanoic acid-1, 8-Naphthalimide (a known compound (3g, 6.15 mmol) was dissolved in anhydrous methanol (60 mL), added K2CO3 (5g, 36.25 mmol), and heated to reflux for 10 hours. After the reaction was completed, filter and wash with dichloromethane After washing 3 times, the organic phases were combined and the solvent was removed under reduced pressure, followed by column chromatography (methanol / dichloromethane=1 / 20, v / v) to obtain a light yellow solid with a yield of 80%. 1HNMR (400 MHz, d6-DMSO, such as Image 6 shown): δ 12.018(s, 1H), 8.479 (d, J = 6.8 Hz, 1H), 8.450 (d, J = 6.0 Hz, 1H), 8.409 (d, J = 6.8 Hz, 1H), 7.771 ( t, J = 6.0 Hz, 1H), 7.283 (d, J = 6.8 Hz, 1H), 4.117 (s, 3H), 3.993 (t, J = 6.0 Hz, 2H), 2.223 (T, J = 6.0 Hz, 2H), 1.617 (m, 2H), 1.556 (m, 2H), 1.346 (m, 2H); 13C NMR (100 MHz, d6-DMSO): δ 174.96, 163.96, 163.32, 160.73, 133.66, 131.42, 128.94, 128.63,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com